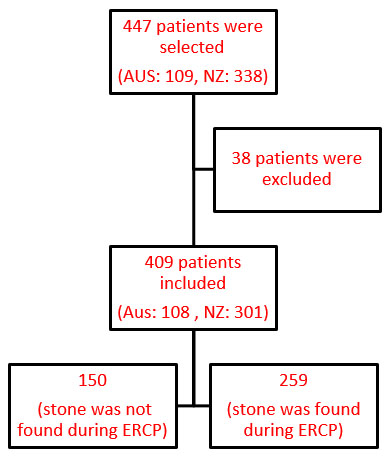
|
Review Article
The effect of genetics and biochemistry on the pathogenesis of cholangiocarcinoma
1 Department of Internal Medicine, Hacettepe University, Faculty of Medicine, Sihhiye, Ankara 06100, Turkey
2 Department of Medical Biochemistry, Hacettepe University, Faculty of Medicine, Sihhiye, Ankara 06100, Turkey
Address correspondence to:
Basak Celtikci
MD, PhD, Assistant Professor, Department of Medical Biochemistry, Hacettepe University, Faculty of Medicine, Sihhiye, Ankara 06100,
Turkey
Message to Corresponding Author
Article ID: 100104Z04MU2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Ucdal M, Burus A, Celtikci B. The effect of genetics and biochemistry on the pathogenesis of cholangiocarcinoma. Int J Hepatobiliary Pancreat Dis 2024;14(2):1–14.ABSTRACT
Cholangiocarcinoma (CCA) presents a significant therapeutic challenge due to its poor prognosis and the complex interplay of metabolic pathways in its development. This study aims to elucidate the genetic, biochemical, and metabolic factors contributing to CCA’s pathogenesis to inform more targeted and effective treatment strategies. A comprehensive review of the current literature was conducted, focusing on the role of genetic variations and metabolic disruptions in CCA. Key pathways such as PI3K/AKT/mTOR, FGFR, and IDH were examined, along with their impacts on carbohydrate, lipid, nucleic acid, and amino acid metabolism. The findings indicate that the liver’s vital role in regulating these metabolic processes means that disruptions can profoundly influence disease progression. Genetic variations were found to significantly alter both metabolic and signaling pathways, contributing to the aggressive nature of CCA. Understanding the complexities of genetic and metabolic interplay in CCA is essential for developing more targeted and effective treatment strategies. This review highlights the importance of these pathways in the pathogenesis of CCA and suggests potential therapeutic targets for future research.
Keywords: Biochemistry, Biomarkers, Cholangiocarcinoma, Genetics, Therapeutic targets
INTRODUCTION
I. Genetic Variations and Biochemical Pathways in Cholangiocarcinoma
This investigation elucidated the pivotal role of genetic variations in modulating metabolic and signaling pathways, contributing to the pathogenesis of cholangiocarcinoma, thereby highlighting the need to comprehend the molecular mechanisms underpinning this malignancy for the development of targeted therapeutic interventions.
II. Impact of FGFR Mutations and PI3K/AKT/mTOR Pathway Alterations
The study highlights the significance of FGFR mutations and alterations in the PI3K/AKT/mTOR pathway in the development of cholangiocarcinoma, proposing these anomalies as potential biomarkers for diagnostic purposes and as targets for therapeutic intervention.
III. Epigenetic Modifications in Disease Progression
The study explored the crucial role of epigenetic modifications, specifically DNA methylation and histone modification, in disease progression, providing insights into novel epigenetic biomarkers and therapeutic intervention strategies.
IV. Metabolic Reprogramming in Cholangiocarcinoma
The review provides a comprehensive overview of the altered metabolic pathways in cholangiocarcinoma, including those involving glucose, lipids, and amino acids, highlighting metabolic reprogramming as a hallmark of cancer that can be exploited for therapeutic purposes.
V. Interplay Between Genetic, Epigenetic, and Metabolic Pathways
Finally, we underscore the interplay between genetic alterations, epigenetic modifications, and metabolic reprogramming in cholangiocarcinoma, suggesting a multifaceted approach for future research and treatment strategies.
Cholangiocarcinomas (CCAs) affect the biliary tree. They make up 10–25% of all primary liver tumors and 3% of all malignancies in the gastrointestinal system. The average age at presentation is 50 years, with Southeast Asia having the highest overall prevalence. Although several risk factors, such as primary sclerosing cholangitis and chronic biliary tract inflammation, have been identified, most patients with CCA have no identifiable risk factors [1].
Multiple alterations in at least 32 genes with substantial changes in protein levels have been observed in patients with CCA [2]. Generally, cholangiocarcinoma originates from biliary epithelial cells and involves multiple intracellular signaling pathways in its development. Table 1 lists the types of CCA, their supposed cells of origin, and the intracellular pathways implicated in their pathogenesis. This detailed overview provides insight into the complex molecular mechanisms underlying CCA [3],[4].
The pathogenesis of cholangiocarcinoma (CCA) involves the dysregulation of several key signaling pathways and genetic alterations. These include the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway, NOTCH signaling, mutations in isocitrate dehydrogenase 1–2 (IDH), and alterations in epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER2). Additionally, fibroblast growth factor receptor (FGFR) fusions, disruptions in the PI3K/Phosphatase and Tensin Homolog (PTEN)/AKT/mTOR signaling axis, mutations in the Kirsten rat sarcoma viral oncogene homolog (KRAS) and v-Raf murine sarcoma viral oncogene homolog B (BRAF), Notch overexpression, and changes in noncoding RNA (ncRNAs) are pivotal contributors to CCA development [2]. The Warburg effect has been shown in CCA but represents a different metabolic pattern than hepatocellular carcinoma (HCC) via downregulated lipid synthesis [5]. The pathways involved in the pathogenesis of CCA are listed in Table 2.
1. The Signaling Pathways in the Pathophysiology of CCA
The most frequently altered pathways in CCA include the PI3K/AKT/mTOR pathway, NOTCH signaling, isocitrate dehydrogenase 1–2, EGFR, FGFR, and HER2 [6].
1.1. FGFR Signaling Pathways in CCA
Fibroblast growth factor receptors (FGFRs), which encompass four tyrosine kinase receptors (FGFR1–4), play crucial roles in cell proliferation, differentiation, and survival. These receptors contain an extracellular domain for ligand binding, a transmembrane helix, and an intracellular kinase domain. Ligand engagement leads to FGFR dimerization and phosphorylation, triggering downstream signaling through pathways such as Ras/RAF/MEK/ERK, JAK/STAT, or PI3K/AKT/mTOR. Additionally, FGFR5, lacking a kinase domain and recently identified, may act in concert with FGFR1 as a co-receptor, further diversifying the biological impact of the FGFR family [7].
1.1.1. FGFR Mutations and Fusions in CCA
Fibroblast growth factor receptor fusions, which are chimeric proteins produced by abnormal chromosomal translocations of FGFR, cause aberrant signaling and consequent dysplasia. Around 15–20% of intrahepatic CCA (iCCA) cases have clinically significant FGFR fusion [8],[9]. Fusion oncogenes and fusions involving FGFR2 represent a new “class” of therapeutic targets in cancer, particularly in CCA [10]. Approximately 10–15% of individuals with CCA have these fusions. Notably, they are almost exclusively seen in iCCA, but not in HCC or perihilar/extrahepatic CCA (eCCA) [11]. Fusions involving additional FGFR family members are uncommon in biliary tract malignancies, with a frequency of less than 0.5% [8],[11].
Although FGFR2 mutations are predominant in CCA, alterations in FGFR1 and , as well as overexpression of FGFR4, also play a remarkable role in the development of the disease. FGFR1 and FGFR3 mutations have been identified in several cases and contribute to the complexity of the genetic landscape of CCA. FGFR4 overexpression has been linked to the increased proliferation and invasion of CCA cells, as demonstrated by in vitro post-stimulation with FGF19 [12]. This suggests that similar to FGFR2, other FGFR family members could be potential targets for therapeutic interventions in CCA. Identification of these mutations and their overexpression highlights the need for a broader focus on the research and treatment of CCA, extending beyond FGFR2.
In addition to FGFR fusions, protein kinase cAMP-activated catalytic subunit beta (PRKACB) rearrangements, such as ATPase Na+/K+ transporting subunit beta 1(ATP1B1)-PRKACB and LINC00261-PRKACB, have also been detected by whole-genome sequencing (WGS) analysis [13]. The PRKACB pseudokinase domain was preserved in both PRKACB rearrangements, which may boost protein kinase A (PKA) activity and activate downstream mitogen activated protein kinase (MAPK) signaling [13].
1.1.2. Prognostic Implications of FGFR Alterations in Cholangiocarcinoma
The presence of FGFR2 fusions in CCA has remarkable prognostic implications. Studies have shown that these genetic alterations are associated with distinct clinical characteristics and responses to targeted therapies. For instance, FGFR2 fusions are more commonly observed in iCCA, nearly exclusive to this subtype, and not typically seen in HCC or eCCA [14]. Although there is preliminary evidence that FGFR2 genetic changes are more common in younger individuals and linked to leisurely disease progression, it is unclear if patients with FGFR2 fusions form a unique prognostic grouping [15]. In conclusion, future FGFR research in CCA should expand beyond FGFR to explore the roles and therapeutic potential of other FGFR family members.
1.2. PI3K/AKT/mTOR Pathway in CCA
The PI3K/AKT/mTOR signaling axis, comprising PI3K, AKT, and mTOR, is pivotal for the onset and progression of CCA. Activation of PI3K leads to the phosphorylation and subsequent activation of AKT, culminating in mTOR activation. Downstream effects of the mTOR pathway are associated with PIK3CA/B activity, illustrating its critical role in the pathophysiology of CCA [16]. The PI3K/AKT/mTOR pathway is essential for regulating key cellular functions such as survival, growth, and cell cycle progression and serves as a fundamental mechanism in cell metabolism and viability. In CCA, alterations such as mutations, changes in gene copy numbers, and irregular protein phosphorylation involving PI3K, AKT, mTOR, and PTEN have been identified and correlated with adverse survival rates [17]. Approximately 30% of the patients exhibit mutations in the PI3K/PTEN/AKT/mTOR signaling pathway [3].
Preclinical studies have indicated that targeting the PI3K/AKT/mTOR pathway with inhibitors has the potential to treat CCA, as demonstrated in both laboratory and animal models. Early clinical trials using rapamycin analogs and mTOR inhibitors have revealed promising efficacy and manageable toxicity. Nevertheless, investigations on AKT and PI3K inhibitors have highlighted safety issues, underscoring the importance of careful patient selection and enhanced specificity in the development of these therapeutic agents [18]. Additionally, concurrent targeting of the PI3-KAKT-mTOR and RAF-MEK-ERK signaling pathways has demonstrated a synergistic effect in CCA, even in overcoming resistance to MEK inhibitors. This finding supports the potential of combining inhibitors of these pathways as a promising strategy for treating patients with CCA in future therapeutic approaches [19].
1.3. RAF-MEK-ERK Pathway in CCA
The RAF-MEK-ERK signaling cascade plays a pivotal role in controlling essential cell functions, such as proliferation, differentiation, and programmed cell death. The activation of this pathway begins with ligand attachment to receptor tyrosine kinases (RTKs), followed by signal relay through a series of protein complexes, including RAS-RAF, MEK, and ERK. Its enrichment in CCA indicates its notable involvement in disease progression [20].
Rat sarcoma virus (RAS), a crucial member of the RAF-MEK-ERK pathway, has notable clinical implications for CCA, particularly through mutations in the KRAS gene. In vitro studies have shown that KRAS mutations in iCCA cells are associated with increased cell growth and migration and reduced E-cadherin expression, which is indicative of enhanced cell motility and the potential for increased metastasis. Furthermore, KRAS mutations have been associated with changes in the adhesion status of iCCA cells and alterations in the responsiveness of tumor cells to interferon immune signals [21].
Additionally, activating KRAS mutations, which affect 25–30% of iCCA subtypes, increases RAF-MEK-ERK signaling [2]. Kirsten rat sarcoma viral oncogene homolog mutations have been linked to worse overall survival in patients with iCCA and are associated with the development of extrahepatic metastases [22]. These mutations are associated with perineural invasion and poor post-operative survival in patients with iCCA [23],[24]. In addition to serving as an indicator of poor prognosis, the presence of KRAS mutations in CCA may considerably influence therapeutic approaches. Cells harboring RAS mutations are predisposed to malignant transformation and display malignant phenotypes that potentially influence their responsiveness to specific treatments [25]. For example, the multikinase inhibitor sorafenib, which targets the MAPK pathway, has been proposed as a potential therapeutic option for CCA [26].
1.4. IDH Mutations in CCA
Isocitrate dehydrogenase is an enzyme involved in the Krebs cycle and exists in two isoforms: IDH1 and IDH2 [27]. Isocitrate dehydrogenase 1/2 normally converts isocitrate to alpha-ketoglutarate, resulting in nicotinamide adenine dinucleotide (NADH). Mutant enzymes convert isocitrate into 2-hydroxy glutarate (2-HG), an oncometabolite that impairs the development of hepatic progenitors and is linked to DNA hypermethylation [28],[29]. These mutations are associated with generation of the oncometabolite R-2-hydroxyglutarate, which can inhibit enzymes that regulate epigenetics, DNA repair, metabolism, and other processes [30]. Isocitrate dehydrogenase mutations are present in 10–30% of patients with iCCA [24]. These mutations are present in 10–20% of patients with iCCA, and up to 90% of these are IDH1 mutations, with only 10–20% being IDH2 mutations [31],[32].
1.5. Therapeutic Potential of IDH Mutations in CCA
Clinical trials have demonstrated that the IDH1 inhibitor ivosidenib offers clinical advantages for patients with IDH1-mutated iCCA, enhancing both progression-free survival and overall survival relative to placebo [33]. The European Society for Medical Oncology (ESMO) endorses the use of ivosidenib in previously treated patients with iCCA with IDH1 mutations [34]. Isocitrate dehydrogenase 1 inhibitors do not benefit all patients, with responders often experiencing disease stabilization rather than notable tumor shrinkage. This indicates the need for deeper insights into the cancer-promoting roles of mutant IDH to develop more effective treatment strategies. Future studies should explore synthetic lethal approaches that leverage the unique cellular conditions induced by mutant IDH1, instead of directly targeting the mutant enzyme itself [35].
Mutations in IDH1 have been linked to immune suppression through the inactivation of TET2, indicating the potential efficacy of combining mutant IDH1 inhibitors with immune checkpoint blockade therapies that target regulatory T cells [36] . In a subset of human CCAs, co-occurring IDH1 and KRAS mutations promote the proliferation of liver progenitor cells, formation of premalignant biliary lesions, and evolution toward metastatic CCA [37]. These findings suggest that combination therapies targeting IDH1 and KRAS mutations may be beneficial.
1.6. EGFR/HER2 Pathway in CCA
Additionally, iCCA tumorigenesis is driven by the EGFR/HER2 pathway. Abnormal regulation of EGFR signaling is the most notable driver mutation. Activating mutations downstream of EGFR, PI3K/PTEN/AKT, and MAPK pathways has also been described [38],[39]. The most frequently treated tumors for eCCA and gallbladder malignancies may have HER2 mutations [40].
1.7. Notch Signaling in CCA
The Notch signaling pathway is a fundamental and evolutionarily preserved system that plays critical roles in regulating cell differentiation, proliferation, and apoptosis [41]. The Notch signaling pathway involves interactions between multiple receptors and ligands, with Notch1, Notch2, Notch3, and Notch4 being the four recognized receptors that are integral to this system [42].
The upregulation of Notch signaling has been implicated as a key mechanism that facilitates the transformation of mature hepatocytes into CCA cells [43]. Notch1 overexpression is associated with CCA initiation and advancement of CCA. Notch1 is connected to cyclin E, a key regulatory protein in the G1 phase of the cell cycle and has the potential to induce DNA damage [43],[44]. The signaling pathways of Notch1 and Notch2 have been implicated in CCA development, with Jagged1 identified as the critical ligand. The elevation of PIK3CA, AKT, and Jagged1 levels directly enhances Notch2 signaling, contributing to CCA pathogenesis. Conversely, Jagged1 overexpression amplifies Notch2 signaling, whereas therapies targeting Jagged1 suppress this pathway [45],[46],[47].
Current knowledge of the role of Notch3 in CCA development and progression remains sparse. However, aberrations in their regulation, particularly through Notch3 overexpression, are associated with cell dedifferentiation, lymph node metastasis, and reduced survival [48].
Recent studies have demonstrated the anticancer properties of gamma secretase inhibitors (GSi), leading to the development and validation of a GSi responder signature [49]. Gamma secretase inhibitors that target the Notch signaling pathway have shown promise in the initial research phases due to their therapeutic potential. Comprehensive studies are needed to elucidate the intricate molecular interactions facilitated by the Notch pathway, as well as its interplay with other signaling pathways. Advancing our knowledge of these areas is pivotal for the development of targeted therapeutic approaches for CCA [50].
Notch4 signaling is associated with the development of iCCA and is correlated with reduced survival rates [51].
The activation of upstream molecules in the Notch signaling pathway, such as Yes-associated protein (YAP), has been observed. Yes-associated protein, a precursor signaling protein for the AKT and mTOR pathways, facilitates CCA development when co-expressed with AKT. This interaction promotes CCA by initiating Notch signaling through the Notch2 receptor [52].
1.8. Hippo Signaling Pathway in CCA
The Hippo pathway, a core evolutionary mechanism, regulates vital functions, such as organ size, tissue repair, stem cell renewal, and oncogenesis. This pathway hinges on a kinase cascade involving the mammalian sterile 20-like kinases 1 and 2 (MST1/2) and large tumor suppressor kinases 1 and 2 (LATS1/2). These kinases act as molecular switches, modifying and inhibiting the transcriptional coactivators, YAP and transcriptional coactivator with PDZ-binding motif (TAZ). Unphosphorylated YAP and TAZ enter the nucleus and interact with TEA domain transcription factors (TEAD 1–4), thereby influencing gene expression for cell growth and proliferation. However, phosphorylation by LATS1/2 kinases restricts YAP/TAZ to the cytoplasm, marking them for degradation by proteasomes and limiting their influence. Thus, the Hippo pathway maintains a delicate balance between YAP/TAZ activity and degradation, thereby ensuring proper organ function and preventing uncontrolled cell growth [53].
This pathway, particularly involving YAP and TAZ, is critically involved in the development and progression of CCA. For instance, TAZ overexpression in the liver can initiate CCA development, although this occurs with low incidence and long latency. Co-expression of TAZ with the myristoylated AKT (myr-AKT) protooncogene considerably increases tumor frequency, accelerates cancer formation, and results in a high tumor burden after hydrodynamic injections [54].
Additionally, the Hippo signaling pathway remarkably affects the differentiation of liver tumors. Disruption of this pathway leads to nuclear accumulation of YAP and TAZ. However, enhancing this pathway through Lats2 overexpression or inhibiting YAP/TAZ function with a dominant-negative variant of TEAD2 can hinder the onset of hepatocarcinogenesis [47].
The Hippo signaling pathway, utilizing its two separate components, MST1/2-SAV1-WWC1-3 (HPO1) and MAP4K1-7-NF2 (HPO2), plays a pivotal role in controlling LATS1/2 kinases and the transcriptional co-activators YAP and TAZ. When either HPO1 or HPO2 is partially inactivated, YAP/TAZ activation is reduced, leading to increased bile duct growth and HCC development. However, completely turning off both HPO1 and HPO2 components leads to full activation of YAP/TAZ, which accelerates CCA progression and leads to earlier death. This detailed understanding of the function of the Hippo pathway underscores its critical role in liver cancer pathology and opens new possibilities for treatment strategies [55]. The major signaling pathways in CCA are shown in Figure 1.
1.9. The Epigenetic Pathogenesis of CCA
Epigenetic alterations play critical roles in CCA pathogenesis. These alterations, which do not change the DNA sequence, involve reversible modifications that affect the DNA or chromosomal structures. The key among these alterations is DNA methylation, in which methyl groups are added to DNA, often at cytosine bases, leading to changes in gene activity. Histone proteins, around which DNA is bound to form nucleosomes, undergo various covalent modifications such as methylation, acetylation, phosphorylation, and ubiquitination. These modifications alter DNA–histone interactions and influence chromatin structure and gene expression. Additionally, noncovalent modifications change the chromatin structure, affecting DNA accessibility to transcription factors and regulatory proteins. Moreover, ncRNAs regulate gene expression post-transcriptionally, often by interacting with chromatin-modifying complexes [56].
1.10. Non-Coding RNA (ncRNA)
Recent studies have shown that ncRNAs play crucial roles in the development and progression of CCA. The term ncRNA encompasses a broad category of genomic transcription products that are not involved in protein coding. This group includes microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). These genes constitute approximately 98% of the human genome. Although ncRNAs are not directly involved in protein encoding, they possess crucial regulatory roles. They mediate a variety of cellular processes including chromatin remodeling, transcription, post-transcriptional modifications, and signal transduction. Ongoing research on ncRNA functions has revealed their critical involvement in cancer biology. These ncRNAs can function as either oncogenes or tumor suppressors and considerably influence CCA progression [57]. Major tumor suppressor and oncogenic ncRNAs and their related genes and pathways are summarized in Table 3 [4],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69],[70].
2. Recent Developments in Genetic and Epigenetic Markers in CCA
Recent advancements in the field of CCA biomarkers have highlighted various novel candidates that could strongly affect the diagnosis, prognosis, and therapeutic strategies for this malignancy. Genetic and epigenetic markers, such as mutations in the BRCA1 associated protein-1 (BAP1) gene and telomerase reverse transcriptase (TERT) promoter, have emerged as potential indicators for CCA, offering insights into its pathogenesis and prognosis [71],[72]. Aberrations in DNA methylation patterns present another layer of complexity in CCA’s molecular landscape, holding promise for early diagnosis. Furthermore, the role of ncRNAs, especially miRNAs, such as miR-21 and miR-221, and lncRNAs, reflecting their diagnostic and prognostic capabilities in CCA, has gained attention [73],[74].
Protein-based markers, which extend beyond conventional markers, have been identified as potential biomarkers relevant to the unique tumor microenvironment of CCA, particularly focusing on cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) [75],[76].
The use of circulating tumor DNA (ctDNA) in liquid biopsies is a noninvasive approach for detecting genetic and epigenetic alterations, paving the way for real-time disease monitoring and personalized treatment approaches [77]. Finally, the adoption of radiomics analysis offers a novel perspective by quantifying tumor characteristics from imaging data, potentially aiding the prediction of treatment responses and disease progression [78].
The convergence of these biomarkers signifies a pivotal shift in CCA management toward more precise and personalized interventions. However, their integration into clinical practice requires extensive research and validation to establish their efficacy and reliability.
3. Metabolic Alterations in Pathophysiology of CCA
3.1. Altered Carbohydrate Metabolism in CCA
Glucose metabolism is one of the major metabolic pathways altered in the onset and progression of CCA. The Warburg effect has been observed in CCA cells, with increased aerobic glycolysis to meet metabolic demands. Therefore, CCA cells depend strictly on glucose uptake. Accordingly, upregulated GLUT-1 expression is correlated with poor prognosis, whereas GLUT-1 silencing reduces CCA cell invasion [79]. The glycolytic enzymes Hexokinase II and pyruvate kinase M2 are upregulated in CCA, as is lactate dehydrogenase (LDH)-A, which converts pyruvate to lactate [80],[81],[82]. By inhibiting pyruvate dehydrogenase (PDHA1) and promoting glycolysis, pyruvate dehydrogenase kinase 1 (PDK1) overexpression has also been observed in CCA, with increased cell proliferation. Interestingly, SIRT3 inhibits the HIF1α/PDK1/ DHA1 pathway, and the reduced SIRT3 expression found in CCA suggests that SIRT3 could exert an anti-Warburg effect in CCA tumorigenesis [83].
Another essential pathway in CCA cells is the pentose phosphate pathway (PPP), which produces ribose-5-phosphate to maintain nucleotide synthesis in rapidly proliferating cells. Pentose phosphate pathway not only sustains nucleotide synthesis but also increases the antioxidant capacity of CCA cells, leading to cisplatin resistance [84].
Other major dysregulated members of glucose-related metabolism are upregulated SIRT2, uncoupling protein 2 (UCP2), and peroxisome proliferator-activated receptor-α (PPAR-α) and downregulated farnesoid X receptor (FXR) as well as IDH1/2 mutations, as mentioned previously [5].
Recently, it is implied that in the tumor microenvironment, CCA cells crosstalk with stromal cells metabolically. For instance, lymphatic endothelial cells (LECs) generate high levels of C-X-C Motif Chemokine Ligand 5 (CXCL5), which communicates with CCA cells via the receptor CXCR2, thereby promoting glucose uptake and lactate production [85]. It is important to elucidate these metabolic communications in order to identify potential targets for CCA treatment. The overall changes in glucose metabolism are shown in Figure 2 [86].
3.2. Altered Lipid Metabolism in CCA
In many carcinomas, including CCA, fatty acid (FA) synthesis and de novo lipogenesis are reported to be upregulated mainly because of the increased expression of fatty acid synthase (FASN) [87]. With this metabolic alteration, tumors no longer rely on exogenous lipid uptake from the bloodstream to promote tumor growth. Interestingly, unlike HCC, iCCA represents a different metabolic pattern with downregulated FASN expression. Accordingly, iCCA cells express high levels of proteins related to FA uptake, such as the FA transporter solute carrier family 27 member 1 (SLC27A1) and fatty acid binding protein 5 (FABP5) [88],[89]. Therefore, while HCC is sensitive to FASN inhibition, CCA is not [88].
Acyl-CoA dehydrogenase (ACADM) overexpression, which occurs in the fatty acid oxidation (FAO) pathway, has also been shown to be directly correlated with CCA proliferation [90]. Studies have noted that cyclooxygenases 2 (COX-2) and prostaglandin E2 (PGE2) levels are upregulated in CCA cells compared to healthy bile duct cells, leading to tumor growth [91]. These findings imply that liver tumors have distinct metabolic features and that the inhibition of exogenous FA uptake and FAO may have a potential role in the therapeutic strategy of human CCAs.
3.3. Altered Amino Acid Metabolism in CCA
Glutamine is a major amino acid involved in CCA metabolism. A study measuring glutamine levels in CCA demonstrated that glutamine deprivation in tumors suppresses proliferation [92]. Furthermore, distant metastasis and poor overall survival correlated with overexpression of glutaminase-1 (GLS1) in iCCA. This suggests that GLS1 is a potential therapeutic target for iCCA [93]. Because CCA cells are dependent on glutamine for proliferation, the expression levels of L-type amino acid transporter 1 (LAT1), a glutamine plasma membrane transporter, are also found to be higher in CCA cells [94]. Thus, LAT1 inhibition may be a potential metabolic target for CCA therapy. Arginine metabolism is altered in CCA with decreased argininosuccinate synthetase (ASS) expression. Considering that ASS occurs during arginine synthesis, CCA tumors are dependent on exogenous arginine uptake due to low arginine levels. This suggests that arginine depletion within tumors results in decreased cell proliferation [95]. Tryptophan hydroxylase expression is increased and monoamine oxidase expression is decreased in CCA, resulting in increased serotonin synthesis. Inhibition of serotonin synthesis suppresses CCA cell growth [96].
The urea cycle is also suppressed in CCA with downregulation of enzymes such as carbamoyl phosphate synthetase 1 (CPS1), which is the rate-limiting enzyme in the urea cycle [97]. Although DNA methylation may suppress CPS1, the mechanism underlying this alteration in the urea cycle remains unclear.
Understanding the altered metabolic pathways underlying CCA is important and could be the basis for new targeted therapeutic approaches. Given the intricate landscape of amino acid metabolism in CCA, where enzymes such as Glutaminase and LAT1 play crucial roles, it is evident that aberrant FGFR signaling can extensively influence these metabolic processes by modulating key pathways, such as MAPK and PI3K/AKT. This modulation may lead to altered activity of these enzymes, thereby affecting the metabolic dependencies and vulnerabilities of CCA cells.
3.4. Altered Signaling Pathways in Lipid and Carbohydrate Metabolism
3.4.1. FGF Pathway
Recent investigations have underscored the role of FGFR mutations, especially FGFR4, in the regulation of lipid and carbohydrate metabolism in the liver, with potential implications for CCA [98]. Fibroblast growth factor receptor 4, a crucial mediator of hepatic bile acid synthesis, has shown particular interest; studies involving liver-specific FGFR4 knockdown in mice on a high-fat diet revealed an increase in bile acid production, reduction in serum cholesterol, and improvements in high-fat diet-induced liver steatosis and insulin resistance, suggesting a pivotal role for FGFR4 in lipid metabolism and the potential development of hepatic steatosis, commonly associated with CCA [99]. Additionally, the Gly385(388) Arg polymorphism in FGFR4 has been identified as a key factor influencing hepatic lipid accumulation and insulin sensitivity under healthy dietary conditions and is linked to increased hepatic triglyceride content, alterations in hepatic lipid composition, and an increase in the expression of genes involved in de novo lipogenesis and glycolysis. Moreover, fibroblast growth factor 21 (FGF21), a hepatokine regulated by FGFR4, has been associated with enhanced lipid profiles and liver function biomarkers, including fibrosis, thereby implicating FGF21 and FGFR4 in lipid metabolism and CCA pathogenesis [98]. However, the precise role of FGFR mutations in lipid and carbohydrate metabolism in CCA is not fully understood and requires further investigation.
It is crucial to recognize that metabolic alterations in CCA are complex and likely involve multiple pathways beyond FGFR signaling, as evidenced by the reliance of highly proliferative human CCA cells on lipid and lipoprotein uptake for fatty acid catabolism, which underscores the need for a comprehensive approach to help in understanding these metabolic changes in CCA [90].
3.4.2. PI3K/AKT/mTOR Pathway
The PI3K/AKT/mTOR signaling cascade is instrumental in reprogramming the metabolic activities of cancer cells, with a pronounced impact on lipid and carbohydrate metabolism. Alterations in this pathway are a common feature of various human cancers, leading to modified patterns of nutrient absorption and consumption, including glucose, glutamine, nucleotides, and lipids. These adjustments facilitate the rapid expansion and proliferation of tumor cells.
In the context of lipid metabolism, this pathway notably affects FAs biosynthesis and breakdown. Specifically, the mTORC1 complex plays a critical role in controlling lipid biosynthesis by acting on sterol regulatory element-binding protein (SREBP) transcription factors. These factors are pivotal in modulating the expression of genes involved in fatty acid and cholesterol production. Cancer cells exhibit an increased uptake and biosynthesis of FAs, coupled with a reduction in their oxidation. This metabolic shift not only generates essential energy in the form of ATP and NADH but also lays the groundwork for the structural integrity of cancer cells, which is essential for their survival and proliferation [100].
Within the domain of carbohydrate metabolism, the PI3K/AKT/mTOR signaling pathway exerts a remarkable influence on glycolysis, notably through the upregulation of glucose transporters such as GLUT1, which is notably increased in expression within cancerous cells. This pathway also plays a crucial role in regulating essential glycolytic enzymes. These include facilitating the phosphorylation of glucose into glucose 6-phosphate by hexokinases, transforming fructose-6-phosphate into fructose-1,6-bisphosphate via phosphofructokinase 1, and converting phosphoenolpyruvate into pyruvate via pyruvate kinase. Such enhancement of glycolytic activity provides cancer cells with a swift supply of energy and necessary metabolic intermediates for their growth and division [101].
In summary, the role of the PI3K/AKT/mTOR pathway in reprogramming cancer cell metabolism is complex and multifaceted, affecting various enzymes and processes crucial for the metabolic adaptations observed in cancer cells.
3.4.3. RAF-MEK-ERK Pathway
The RAF-MEK-ERK pathway, including the RAS subpathway, plays a remarkable role in metabolic reprogramming observed in CCA. This reprogramming can involve both lipid and carbohydrate metabolism. The RAS gene, frequently mutated and abnormally activated in cancers, can regulate lipid metabolism through the AKT-mTORC1 axis or other pathways, thereby affecting lipid synthesis and degradation. This regulation influences the growth and survival of cancer cells, making them potential targets for antitumor therapy.
In addition to lipid metabolism, the RAS pathway influences carbohydrate metabolism. Although the specific mechanisms are complex and not fully understood, the RAS pathway promotes glycolysis, a key aspect of carbohydrate metabolism that is often upregulated in cancer cells [100].
3.5. Altered Signaling Pathways in Amino Acid Metabolism
3.5.1. FGF Pathway
In CCA, the aberrant FGFR signaling notably affects amino acid metabolism, which is a crucial aspect of cancer cell proliferation and survival. This effect is primarily mediated by the MAPK and PI3K/AKT pathways, which are triggered via FGFR activation. Within the MAPK pathway, enzymes such as RAS, RAF, MEK, and ERK play pivotal roles. Rat sarcoma virus activates RAF, which in turn phosphorylates MEK, leading to the activation of ERK. This cascade ultimately results in the regulation of the transcription of genes related to amino acid metabolism.
In parallel, the PI3K/AKT pathway, initiated by FGFR signaling, involves PI3K converting PIP2 to PIP3 and subsequently activating AKT. Protein Kinase B activity affects various metabolic processes, including activation of mTOR, a master regulator of cell metabolism. Mammalian target of rapamycin controls protein synthesis by influencing the activity of enzymes, such as S6K and 4E-BP1. Moreover, in amino acid metabolism, enzymes such as glutaminases, which convert glutamine to glutamate, and transaminases, such as aspartate aminotransferase and alanine aminotransferase, are crucial. These enzymes are involved in amino acid synthesis and degradation and are potentially dysregulated in CCA due to altered FGFR signaling [12],[102],[103]. Understanding the intricate roles of these enzymes in FGFR-driven pathways underscores the complex interplay between cancer signaling and metabolic regulation, highlighting potential targets for therapeutic interventions in CCA.
3.5.2. PI3K/AKT/mTOR Pathway
Amino acids activate mTORC1 and hVps34 in cancer cells through an increase in intracellular calcium, which enhances Ca2+/calmodulin binding to hVps34, boosting lipid kinase activity and mTORC1 signaling. This pathway underscores the crucial link between metabolic disturbances and cancer progression and offers potential targets for cholangiocarcinoma treatment [104].
Preclinical studies have shown that combined targeting of mTOR and AKT using RAD001 (an mTOR inhibitor) and MK-2206 (an AKT inhibitor) is synergistic in the treatment of CCA. This suggests that the combined targeting of mTOR and AKT may be a new and effective strategy for the treatment of CCA. However, further clinical trials are needed to validate these preclinical findings and determine the optimal doses of RAD001 and MK-2206 for the treatment of CCA [18].
Frequent dysregulation of the PI3K/AKT/mTOR pathway has been observed in CCA, contributing to disease progression and resistance to therapies [105]. Therefore, targeting this pathway, including the specific mechanisms by which it regulates amino acid synthesis, may have therapeutic potential for CCA.
3.5.3. Notch Pathway
The Notch pathway has been implicated in the regulation of amino acid metabolism, particularly through its interaction with mammalian target of mTORC1 signaling pathway. The Notch pathway, specifically through the atypical receptor NOTCH3, promotes tumor cell survival via activation of the PI3KAKT pathway. This pathway regulates cellular processes such as growth and metabolism, including amino acid metabolism. Furthermore, the Notch pathway interacts with mTORC1, a central regulator of cell metabolism, growth, proliferation, and survival. The mTORC1 pathway is sensitive to amino acid availability and is activated in response to amino acid abundance. In CCA, simultaneous activation of the Notch and mTORC1 pathways has been observed, leading to increased expression of amino acid transporters and enhanced tumor growth [48],[106].
Additionally, when activated along the KRAS-AKT pathway, the Notch pathway induces biliary cancer development via the mTORC1 pathway. This suggests a complex interplay between the Notch pathway, other signaling pathways, and amino acid metabolism in CCA [107].
In summary, the Notch pathway plays a crucial role in CCA, partly through its influence on amino acid metabolism through interactions with the mTORC1 pathway. This highlights the potential of targeting these pathways for therapeutic interventions in CCA.
3.5.4. Hippo Pathway
The Hippo signaling pathway comprises two critical modules, HPO1 and HPO2, which govern the functionality of LATS1/2 kinases and YAP/TAZ transcriptional co-activators. These elements play pivotal roles in modulating alterations in organ dimensions and pathways leading to tumorigenesis, underscoring their significance in cellular growth and cancer development. Within the framework of amino acid metabolism, the effectors of the Hippo pathway, namely YAP and TAZ, play a pivotal role in regulating amino acid signals towards mTORC1, an essential modulator of cellular proliferation and metabolic processes. Through its interaction with TEAD transcription factors, YAP and TAZ facilitate the upregulation of LAT1, a high-affinity leucine transporter. This transporter is integral for leucine assimilation and subsequent mTORC1 activation, especially in nutrient scarcity scenarios, and research indicates the notable involvement of the Hippo pathway in the onset of iCCA. Genetic deactivation of the HPO1 and HPO2 signaling modules triggers the complete activation of YAP/TAZ, precipitating the accelerated progression of iCCA [55],[108].
In summary, the Hippo signaling pathway plays a remarkable role in amino acid metabolism, primarily through the regulation of mTORC1 activation via YAP/TAZ and LAT1. It is also involved in the pathogenesis of CCA, and alterations in this pathway contribute to iCCA development.
Conclusion
The intricate relationship between genetics and biochemistry plays a pivotal role in the pathogenesis of cholangiocarcinoma, which is a complex and multifaceted liver disease. Recent advances in WGS have extensively enhanced our understanding of how genetic variation influences biochemical pathways and contributes to disease development and progression. This review focuses on dissecting the crosstalk between genetic variations and biochemical pathways in cholangiocarcinoma. We explored key signaling pathways such as RAF/MEK/ERK and PI3K/AKT/mTOR, which are crucial in tumorigenesis and cellular metabolism. These pathways not only offer insights into the molecular mechanisms of the disease but also present potential targets for novel diagnostic and prognostic biomarkers and therapeutic interventions. Furthermore, this review highlights newly identified genetic variants and their impact on biochemical pathways, underscoring the importance of genetic diversity in disease manifestation. By exploring the intricate interplay between genetics and biochemistry, we aimed to provide a comprehensive understanding of the regulatory mechanisms of hepatocellular homeostasis and their implications in cholangiocarcinoma pathogenesis
REFERENCES
1.
Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54(1):173–84. [CrossRef]
[Pubmed]

2.
Sohal DPS, Shrotriya S, Abazeed M, Cruise M, Khorana A. Molecular characteristics of biliary tract cancer. Crit Rev Oncol Hematol 2016;107:111–8. [CrossRef]
[Pubmed]

3.
Chakrabarti S, Kamgar M, Mahipal A. Targeted therapies in advanced biliary tract cancer: An evolving paradigm. Cancers (Basel) 2020;12(8):2039. [CrossRef]
[Pubmed]

4.
Wang N, Zhang C, Wang W, et al. Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis 2019;10(8):585. [CrossRef]
[Pubmed]

5.
Pastore M, Lori G, Gentilini A, et al. Multifaceted aspects of metabolic plasticity in human cholangiocarcinoma: An overview of current perspectives. Cells 2020;9(3):596. [CrossRef]
[Pubmed]

6.
Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47(9):1003–10. [CrossRef]
[Pubmed]

7.
Regeenes R, Silva PN, Chang HH, et al. Fibroblast growth factor receptor 5 (FGFR5) is a co-receptor for FGFR1 that is up-regulated in beta-cells by cytokine-induced inflammation. J Biol Chem 2018;293(44):17218–28. [CrossRef]
[Pubmed]

8.
Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014;59(4):1427–34. [CrossRef]
[Pubmed]

9.
Presta M, Chiodelli P, Giacomini A, Rusnati M, Ronca R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol Ther 2017;179:171–87. [CrossRef]
[Pubmed]

10.
11.
Javle MM, Murugesan K, Shroff RT, et al. Profiling of 3,634 cholangiocarcinomas (CCA) to identify genomic alterations (GA), tumor mutational burden (TMB), and genomic loss of heterozygosity (gLOH). J Clin Oncol 2019;37(15 suppl):4087. [CrossRef]

12.
Liu Y, Cao M, Cai Y, Li X, Zhao C, Cui R. Dissecting the role of the FGF19-FGFR4 signaling pathway in cancer development and progression. Front Cell Dev Biol 2020;8:95. [CrossRef]
[Pubmed]

13.
Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518(7540):495–501. [CrossRef]
[Pubmed]

14.
Chmiel P, Gęca K, Rawicz-Pruszyński K, Polkowski WP, Skórzewska M. FGFR inhibitors in cholangiocarcinoma—A novel yet primary approach: Where do we stand now and where to head next in targeting this axis? Cells 2022;11(23):3929. [CrossRef]
[Pubmed]

15.
Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype. JCO Precis Oncol 2018;2:1–12. [CrossRef]
[Pubmed]

16.
Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2012;28(3):266–72. [CrossRef]
[Pubmed]

17.
Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives. Cancer Treat Rev 2019;72:45–55. [CrossRef]
[Pubmed]

18.
Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer 2023;22(1):138. [CrossRef]
[Pubmed]

19.
Ewald F, Nörz D, Grottke A, Hofmann BT, Nashan B, Jücker M. Dual inhibition of PI3K-AKT-mTOR-and RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK-inhibitors. Invest New Drugs 2014;32(6):1144–54. [CrossRef]
[Pubmed]

20.
Chakraborty J, Chakraborty S, Chakraborty S, Narayan MN. Entanglement of MAPK pathways with gene expression and its omnipresence in the etiology for cancer and neurodegenerative disorders. Biochim Biophys Acta Gene Regul Mech 2023;1866(4):194988. [CrossRef]
[Pubmed]

21.
Li C, He WQ. Comparison of primary liver cancer mortality estimates from World Health Organization, global burden disease and global cancer observatory. Liver Int 2022;42(10):2299–16. [CrossRef]
[Pubmed]

22.
Tanaka M, Kunita A, Yamagishi M, et al. KRAS mutation in intrahepatic cholangiocarcinoma: Linkage with metastasis-free survival and reduced E-cadherin expression. Liver Int 2022;42(10):2329–40. [CrossRef]
[Pubmed]

23.
Chang YT, Chang MC, Huang KW, Tung CC, Hsu C, Wong JM. Clinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in Taiwan. J Gastroenterol Hepatol 2014;29(5):1119–25. [CrossRef]
[Pubmed]

24.
Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy. Ann Surg Oncol 2012;19 Suppl 3:S675–81. [CrossRef]
[Pubmed]

25.
Chen K, Zhang Y, Qian L, Wang P. Emerging strategies to target RAS signaling in human cancer therapy. J Hematol Oncol 2021;14(1):116. [CrossRef]
[Pubmed]

26.
Delire B, Stärkel P. The Ras/MAPK pathway and hepatocarcinoma: Pathogenesis and therapeutic implications. Eur J Clin Invest 2015;45(6):609–23 [CrossRef]
[Pubmed]

27.
Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol 2021;32(9):1111–26. [CrossRef]
[Pubmed]

28.
Saha SK, Parachoniak CA, Bardeesy N. IDH mutations in liver cell plasticity and biliary cancer. Cell Cycle 2014;13(20):3176–82. [CrossRef]
[Pubmed]

29.
Mondesir J, Willekens C, Touat M, de Botton S. IDH1 and IDH2 mutations as novel therapeutic targets: Current perspectives. J Blood Med 2016;7:171–80. [CrossRef]
[Pubmed]

30.
Wu MJ, Shi L, Merritt J, Zhu AX, Bardeesy N. Biology of IDH mutant cholangiocarcinoma. Hepatology 2022;75(5):1322–37. [CrossRef]
[Pubmed]

31.
Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014; 5(9):2839–52. [CrossRef]
[Pubmed]

32.
Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J Hepatol 2020;73(1):170–85. [CrossRef]
[Pubmed]

33.
Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21(6):796–807. [CrossRef]
[Pubmed]

34.
Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34(2):127–40. [CrossRef]
[Pubmed]

35.
Lavacchi D, Caliman E, Rossi G, et al. Ivosidenib in IDH1-mutated cholangiocarcinoma: Clinical evaluation and future directions. Pharmacol Ther 2022;237:108170. [CrossRef]
[Pubmed]

36.
Zhu Y, Kwong LN. IDH1 inhibition reawakens the immune response against cholangiocarcinoma. Cancer Discov 2022;12(3):604–5. [CrossRef]
[Pubmed]

37.
Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF-4? to block hepatocyte differentiation and promote biliary cancer. Nature 2014;513(7516):110–4. [CrossRef]
[Pubmed]

38.
Leone F, Cavalloni G, Pignochino Y, et al. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res 2006;12(6):1680–5. [CrossRef]
[Pubmed]

39.
Gwak GY, Yoon JH, Shin CM, et al. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J Cancer Res Clin Oncol 2005;131(10):649–52. [CrossRef]
[Pubmed]

40.
Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98(2):418–25. [CrossRef]
[Pubmed]

41.
Pancewicz-Wojtkiewicz J. Epidermal growth factor receptor and notch signaling in non-small-cell lung cancer. Cancer Med 2016;5(12):3572–8. [CrossRef]
[Pubmed]

42.
Kandasamy K, Mohan SS, Raju R, et al. NetPath: A public resource of curated signal transduction pathways. Genome Biol 2010;11(1):R3. [CrossRef]
[Pubmed]

43.
Zender S, Nickeleit I, Wuestefeld T, et al. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell 2013;23(6):784–95. [CrossRef]
[Pubmed]

44.
Ding N, Che L, Li XL, et al. Oncogenic potential of IDH1R132C mutant in cholangiocarcinoma development in mice. World J Gastroenterol 2016;22(6):2071–80. [CrossRef]
[Pubmed]

45.
Huntzicker EG, Hötzel K, Choy L, et al. Differential effects of targeting Notch receptors in a mouse model of liver cancer. Hepatology 2015;61(3):942–52. [CrossRef]
[Pubmed]

46.
Che L, Fan B, Pilo MG, et al. Jagged 1 is a major Notch ligand along cholangiocarcinoma development in mice and humans. Oncogenesis 2016;5(12):e274. [CrossRef]
[Pubmed]

47.
Zhang S, Wang J, Wang H, et al. Hippo cascade controls lineage commitment of liver tumors in mice and humans. Am J Pathol 2018;188(4):995–1006. [CrossRef]
[Pubmed]

48.
Guest RV, Boulter L, Dwyer BJ, et al. Notch3 drives development and progression of cholangiocarcinoma. Proc Natl Acad Sci U S A 2016;113(43):12250–55. [CrossRef]
[Pubmed]

49.
O’Rourke CJ, Matter MS, Nepal C, et al. Identification of a pan-gamma-secretase inhibitor response signature for notch-driven cholangiocarcinoma. Hepatology 2020;71(1):196–213. [CrossRef]
[Pubmed]

50.
Rauff B, Malik A, Bhatti YA, Chudhary SA, Qadri I, Rafiq S. Notch signalling pathway in development of cholangiocarcinoma. World J Gastrointest Oncol 2020;12(9):957–74. [CrossRef]
[Pubmed]

51.
Wu WR, Shi XD, Zhang R, et al. Clinicopathological significance of aberrant Notch receptors in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol 2014;7(6):3272–9.
[Pubmed]

52.
Yamamoto M, Xin B, Watanabe K, et al. Oncogenic determination of a broad spectrum of phenotypes of hepatocyte-derived mouse liver tumors. Am J Pathol. 2017;187(12):2711–25. [CrossRef]
[Pubmed]

53.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 2010;24(1):72–85. [CrossRef]
[Pubmed]

54.
Cigliano A, Zhang S, Ribback S, et al. The Hippo pathway effector TAZ induces intrahepatic cholangiocarcinoma in mice and is ubiquitously activated in the human disease. J Exp Clin Cancer Res 2022;41(1):192. [CrossRef]
[Pubmed]

55.
Qi S, Zhong Z, Zhu Y, et al. Two Hippo signaling modules orchestrate liver size and tumorigenesis. EMBO J 2023;42(11):e112126. [CrossRef]
[Pubmed]

56.
O’Rourke CJ, Lafuente-Barquero J, Andersen JB. Epigenome remodeling in cholangiocarcinoma. Trends Cancer 2019;5(6):335–50. [CrossRef]
[Pubmed]

57.
Yan H, Bu P. Non-coding RNA in cancer. Essays Biochem 2021;65(4):625–39. [CrossRef]
[Pubmed]

58.
Zhang Y, Zhang L, Lu S, et al. Long non-coding RNA CASC15 promotes intrahepatic cholangiocarcinoma possibly through inducing PRDX2/PI3K/AKT axis. Cancer Res Treat 2021;53(1):184–98. [CrossRef]
[Pubmed]

59.
Xu Y, Yao Y, Jiang X, et al. SP1-induced upregulation of lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangiocarcinoma. J Exp Clin Cancer Res 2018;37(1):81. [CrossRef]
[Pubmed]

60.
Jiang ZL, Zhang FX, Zhan HL, et al. miR-181b-5p promotes the progression of cholangiocarcinoma by targeting PARK2 via PTEN/PI3K/AKT signaling pathway. Biochem Genet 2022;60(1):223–40. [CrossRef]
[Pubmed]

61.
Jiang ZL, Zhang FX, Zhan HL, et al. miR-181b-5p promotes the progression of cholangiocarcinoma by targeting PARK2 via PTEN/PI3K/AKT signaling pathway. Biochem Genet 2022;60(1):223–40. [CrossRef]
[Pubmed]

62.
Zhang JW, Wang X, Li GC, et al. MiR-30a-5p promotes cholangiocarcinoma cell proliferation through targeting SOCS3. J Cancer 2020;11(12):3604–14 [CrossRef]
[Pubmed]

63.
Xu YP, Dong ZN, Wang SW, et al. circHMGCS1-016 reshapes immune environment by sponging miR-1236-3p to regulate CD73 and GAL-8 expression in intrahepatic cholangiocarcinoma. J Exp Clin Cancer Res 2021;40(1):290. [CrossRef]
[Pubmed]

64.
Li O, Jiang B, Yi WM, et al. LncRNA NEAT1 promotes cell proliferation, migration, and invasion via the miR-186-5p/PTP4A1 axis in cholangiocarcinoma. Kaohsiung J Med Sci 2021;37(5):379–91. [CrossRef]
[Pubmed]

65.
Ma L, Li T, Liu G, Wang J, Yin Z, Kang J. LncRNA RHPN1-AS1 modulates cholangiocarcinoma progression and is related with poor clinical outcomes through miR-345-5p/YAP1 axis. J Gastroenterol Hepatol 2020;12(16):141–58. [CrossRef]
[Pubmed]

66.
Tang C, Yuan P, Wang J, et al. MiR-192-5p regulates the proliferation and apoptosis of cholangiocarcinoma cells by activating MEK/ERK pathway. 3 Biotech 2021;11(2):99. [CrossRef]
[Pubmed]

67.
Wang D, Xiong F, Wu G, Liu W, Wang B, Chen Y. MiR-155-5p suppresses SOX1 to promote proliferation of cholangiocarcinoma via RAF/MEK/ERK pathway. Cancer Cell Int 2021;21(1):656. [CrossRef]
[Pubmed]

68.
Gao L, Yang X, Zhang H, Yu M, Long J, Yang T. Inhibition of miR-10a-5p suppresses cholangiocarcinoma cell growth through downregulation of Akt pathway. Onco Targets Ther 2018;11:6981–94. [CrossRef]
[Pubmed]

69.
Chen Q, Wang H, Li Z, et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol 2022;76(1):135–47. [CrossRef]
[Pubmed]

70.
Du J, Lan T, Liao H, et al. CircNFIB inhibits tumor growth and metastasis through suppressing MEK1/ERK signaling in intrahepatic cholangiocarcinoma. Mol Cancer 2022;21(1):18. [CrossRef]
[Pubmed]

71.
Yamashita H, Tourna A, Akita M, et al. Epigenetic upregulation of TET2 is an independent poor prognostic factor for intrahepatic cholangiocarcinoma. Virchows Arch 2022;480(5):1077–85. [CrossRef]
[Pubmed]

72.
Rizzo A, Carloni R, Ricci AD, et al. Molecular profile and prognostic value of BAP1 mutations in intrahepatic cholangiocarcinoma: A genomic database analysis. J Pers Med 2022;12(8):1247. [CrossRef]
[Pubmed]

73.
Macias RIR, Kornek M, Rodrigues PM, et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int 2019;39 Suppl 1:108–22. [CrossRef]
[Pubmed]

74.
Zhou QY, Peng PL, Xu YH. MiR-221 affects proliferation and apoptosis of gastric cancer cells through targeting SOCS3. Eur Rev Med Pharmacol Sci 2019;23(21):9427–35. [CrossRef]
[Pubmed]

75.
Kinzler MN, Schulze F, Gretser S, et al. Expression of MUC16/CA125 is associated with impaired survival in patients with surgically resected cholangiocarcinoma. Cancers (Basel) 2022;14(19):4703. [CrossRef]
[Pubmed]

76.
Vaquero J, Aoudjehane L, Fouassier L. Cancer-associated fibroblasts in cholangiocarcinoma. Curr Opin Gastroenterol 2020;36(2):63–69. [CrossRef]
[Pubmed]

77.
Yoo C, Laliotis G, Jeong H, et al. Utility of circulating tumor DNA (ctDNA) as a predictive biomarker for disease monitoring in patients (pts) with cholangiocarcinoma (CCA) before and during adjuvant chemotherapy (ACT): Sub-analysis of the randomized phase 2 STAMP trial. Journal of Clinical Oncology. 2023;41(16_Suppl):4123 [CrossRef]

78.
Fiz F, Rossi N, Langella S, et al. Radiomic analysis of intrahepatic cholangiocarcinoma: Non-invasive prediction of pathology data: A multicenter study to develop a clinical-radiomic model. Cancers (Basel) 2023;15(17):4204. [CrossRef]
[Pubmed]

79.
Kubo Y, Aishima S, Tanaka Y, et al. Different expression of glucose transporters in the progression of intrahepatic cholangiocarcinoma. Hum Pathol 2014;45(8):1610–7. [CrossRef]
[Pubmed]

80.
Qian Z, Hu W, Lv Z, et al. PKM2 upregulation promotes malignancy and indicates poor prognosis for intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol 2020;44(2):162–73. [CrossRef]
[Pubmed]

81.
Yu Y, Liao M, Liu R, Chen J, Feng H, Fu Z. Overexpression of lactate dehydrogenase – A in human intrahepatic cholangiocarcinoma: Its implication for treatment. World J Surg Oncol 2014;12:78. [CrossRef]
[Pubmed]

82.
Thamrongwaranggoon U, Seubwai W, Phoomak C, et al. Targeting hexokinase II as a possible therapy for cholangiocarcinoma. Biochem Biophys Res Commun 2017;484(2):409–15. [CrossRef]
[Pubmed]

83.
Xu L, Li Y, Zhou L, et al. SIRT3 elicited an anti- Warburg effect through HIF1?/PDK1/PDHA1 to inhibit cholangiocarcinoma tumorigenesis. Cancer Med 2019;8(5):2380–91. [CrossRef]
[Pubmed]

84.
Qu X, Sheng J, Shen L, et al. Autophagy inhibitor chloroquine increases sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by mitochondrial ROS. PLoS One 2017;12(3):e0173712. [CrossRef]
[Pubmed]

85.
Roy S, Kumaravel S, Banerjee P, et al. Tumor lymphatic interactions induce CXCR2-CXCL5 axis and alter cellular metabolism and lymphangiogenic pathways to promote cholangiocarcinoma. Cells 2021;10(11):3093. [CrossRef]
[Pubmed]

86.
Raggi C, Taddei ML, Rae C, Braconi C, Marra F. Metabolic reprogramming in cholangiocarcinoma. J Hepatol 2022;77(3):849–64. [CrossRef]
[Pubmed]

87.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007;7(10):763–77. [CrossRef]
[Pubmed]

88.
Li L, Che L, Tharp KM, et al. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology 2016;63(6):1900–13. [CrossRef]
[Pubmed]

89.
Nakagawa R, Hiep NC, Ouchi H, Sato Y, Harada K. Expression of fatty-acid-binding protein 5 in intrahepatic and extrahepatic cholangiocarcinoma: The possibility of different energy metabolisms in anatomical location. Med Mol Morphol 2020;53(1):42–9. [CrossRef]
[Pubmed]

90.
Ruiz de Gauna M, Biancaniello F, González-Romero F, et al. Cholangiocarcinoma progression depends on the uptake and metabolization of extracellular lipids. Hepatology 2022;76(6):1617–33. [CrossRef]
[Pubmed]

91.
Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: Evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res 2004;64(4):1369–76 [CrossRef]
[Pubmed]

92.
Wappler J, Arts M, Röth A, et al. Glutamine deprivation counteracts hypoxia-induced chemoresistance. Neoplasia 2020;22(1):22–32. [CrossRef]
[Pubmed]

93.
Cao J, Zhang C, Jiang GQ, et al. Expression of GLS1 in intrahepatic cholangiocarcinoma and its clinical significance. Mol Med Rep 2019;20(2):1915–24. [CrossRef]
[Pubmed]

94.
Kaira K, Sunose Y, Ohshima Y, et al. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 2013;13:482. [CrossRef]
[Pubmed]

95.
Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 2018;29(6):1402–8. [CrossRef]
[Pubmed]

96.
Alpini G, Invernizzi P, Gaudio E, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res 2008;68(22):9184–93. [CrossRef]
[Pubmed]

97.
Liu H, Dong H, Robertson K, Liu C. DNA methylation suppresses expression of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular carcinoma. Am J Pathol 2011;178(2):652–61. [CrossRef]
[Pubmed]

98.
Larsson SC, Michaëlsson K, Mola-Caminal M, Höijer J, Mantzoros CS. Genome-wide association and Mendelian randomization study of fibroblast growth factor 21 reveals causal associations with hyperlipidemia and possibly NASH. Metabolism 2022;137:155329. [CrossRef]
[Pubmed]

99.
Moreau F, Brunao BB, Liu XY, et al. Liver-specific FGFR4 knockdown in mice on an HFD increases bile acid synthesis and improves hepatic steatosis. J Lipid Res 2023;64(2):100324. [CrossRef]
[Pubmed]

100.
Zhang M, Pan J, Huang P. Interaction between RAS gene and lipid metabolism in cancer. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021;50(1):17–22. [CrossRef]
[Pubmed]

101.
102.
Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell 2017;169(3):381–405. [CrossRef]
[Pubmed]

103.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene 2007;26(22):3279–90. [CrossRef]
[Pubmed]

104.
Gulati P, Gaspers LD, Dann SG, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 2008;7(5):456–65. [CrossRef]
[Pubmed]

105.
Ewald F, Grabinski N, Grottke A, et al. Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int J Cancer 2013;133(9):2065–76. [CrossRef]
[Pubmed]

106.
Lu X, Peng B, Chen G, et al. YAP Accelerates Notch-driven cholangiocarcinogenesis via mTORC1 in mice. Am J Pathol 2021;191(9):1651–67. [CrossRef]
[Pubmed]

107.
Namikawa M, Fukuda A, Mizukoshi K, et al. Simultaneous activation of Kras-Akt and Notch pathways induces extrahepatic biliary cancer via the mTORC1 pathway. J Pathol 2023;260(4):478–92. [CrossRef]
[Pubmed]

108.
Hansen CG, Ng YLD, Lam WLM, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res 2015;25(12):1299–313. [CrossRef]
[Pubmed]

109.
Yu Y, Chen Q, Zhang X, et al. Long noncoding RNA ANRIL promotes the malignant progression of cholangiocarcinoma by epigenetically repressing ERRFI1 expression. Cancer Sci 2020;111(7):2297–309. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Mete Ucdal - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ayse Burus - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Basak Celtikci - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Mete Ucdal et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.