|
Case Report
A unique presentation of peliosis hepatis: A case report and comprehensive review of the literature
1 University of South Carolina School of Medicine Greenville, Greenville, SC, USA
2 Department of Radiology, Prisma Health Upstate, Greenville, SC, USA
3 Pathology Associates, 8 Memorial Medical Ct., Greenville, SC, USA
Address correspondence to:
Christine Marie-Gilligan Schammel
PhD, Director, Clinical Research, Department of Pathology, Prisma Health Upstate, SC, USA; Pathology Associates, 8 Memorial Medical Ct., Greenville, SC 29605,
USA
Message to Corresponding Author
Article ID: 100103Z04AT2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Teshon A, Walker C, Schammel DP, Schammel CMG, Devane AM. A unique presentation of peliosis hepatis: A case report and comprehensive review of the literature. Int J Hepatobiliary Pancreat Dis 2023;13(1):16–25.ABSTRACT
Introduction: Peliosis hepatis (PH) is a rare benign vascular condition characterized by dilatation of hepatic sinusoids with occasional involvement of other organs. While associated with chronic immunosuppression, anabolic steroid use, oral contraceptive (OCP) use, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), and infection with Bartonella, most PH patients are asymptomatic and, thus, identified incidentally. Compromised liver function is sometimes evident through laboratory tests; however, mortality results from cyst rupture and hemorrhage spontaneously or during surgical procedures.
Case Report: We report a case of PH identified in a 33-year-old Black female radiologically evaluated for abnormal liver function tests. Computed tomography (CT) revealed enhancement of >100 lesions throughout both liver lobes; a CT-guided biopsy revealed mild macrovesicular steatosis and marked sinusoidal dilation, consistent with peliosis hepatis.
Conclusion: We also present a comprehensive literature review describing the associated conditions, pathology, diagnostic methods, and treatment options for PH patients.
Keywords: Benign vascular liver condition, Dilation of sinusoidal liver spaces, Peliosis hepatis
Introduction
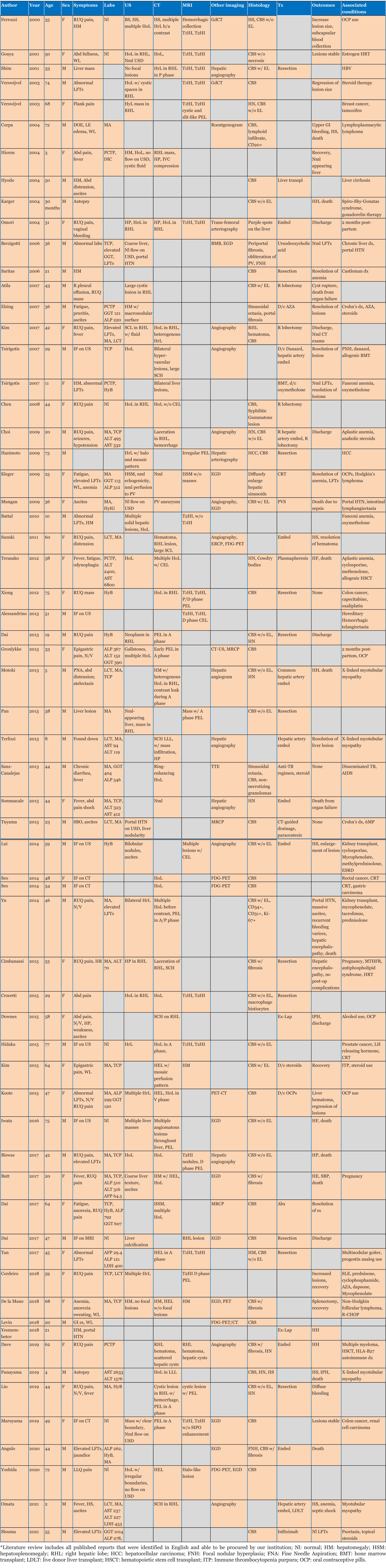
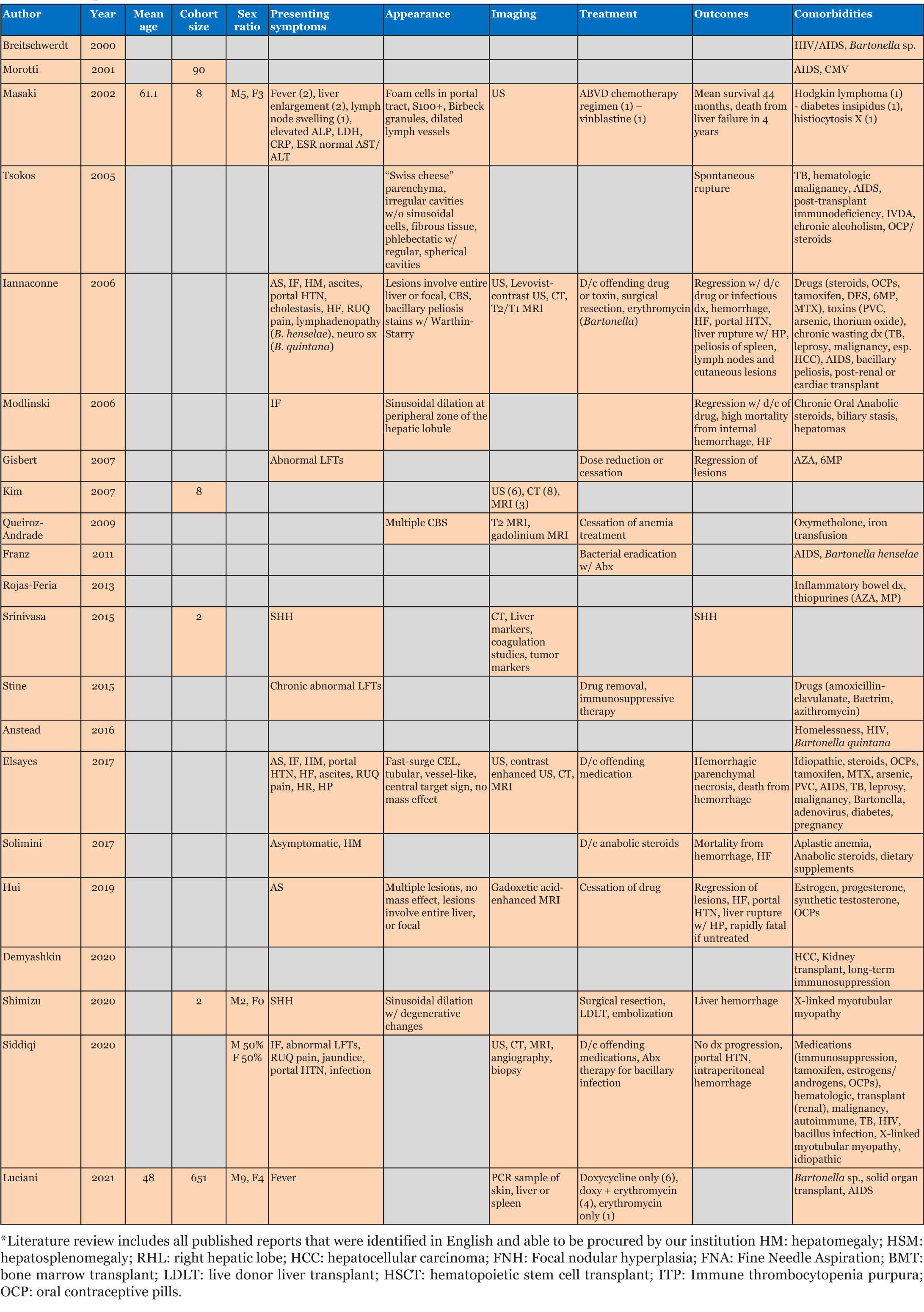
Peliosis hepatis (PH) is a rare benign vascular condition with a reported incidence between 0.2 and 22%, characterized by dilatation of hepatic sinusoids and occasional involvement of other organs [1],[2],[3],[4],[5],[6]. Risk factors include chronic immunosuppression, anabolic steroid use, oral contraceptive (OCP) use, HIV/AIDS, and infection with Bartonella spp [2],[4],[7],[8],[9],[10],[11],[12],[13]. Most cases are discovered incidentally on imaging or during routine follow-ups for abnormal lab results [2],[9],[14],[15],[16],[17],[18]. Peliosis hepatis lesions may spontaneously or surgically rupture and hemorrhage, which is associated with significant mortality [4],[8],[19],[20],[21],[22]. Interestingly, reports note spontaneous regression of lesions with cessation of causative medications, normalizing liver function tests [6],[13],[23]. Peliosis hepatis lesions can be identified by sonography, CT, magnetic resonance imaging (MRI), and angiography. However, the definitive diagnosis is determined by the pathologic pattern of disease, stages of the blood component in the lesions, and hepatic steatosis for differentiation from hepatic adenomas, hemangiomas, hepatic abscesses, or hepatocellular carcinoma [16]. Treatment for persistent PH is resection to avoid progression to cholestasis, portal hypertension [1],[4],[12], and hepatic failure; however, biopsies and resection are associated with significant bleeding risk and mortality [1],[9],[24],[25].
Following Institutional Review Board (IRB) approval and consent, we present radiographic findings of PH in an asymptomatic patient with stable lesions successfully managed with regular radiographic follow-up. Additionally, we present a comprehensive review of the literature describing the pathology, associated conditions, diagnostic methods, and treatment options for PH.
Case Report
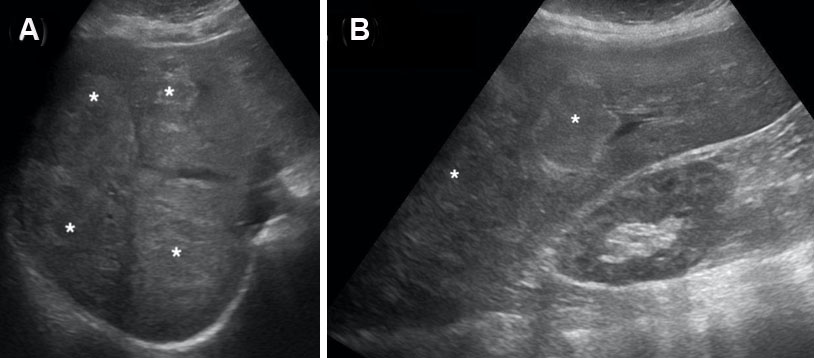
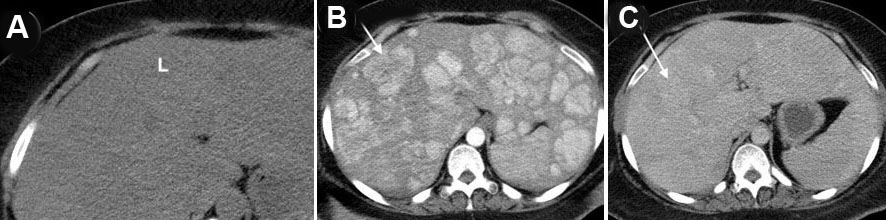
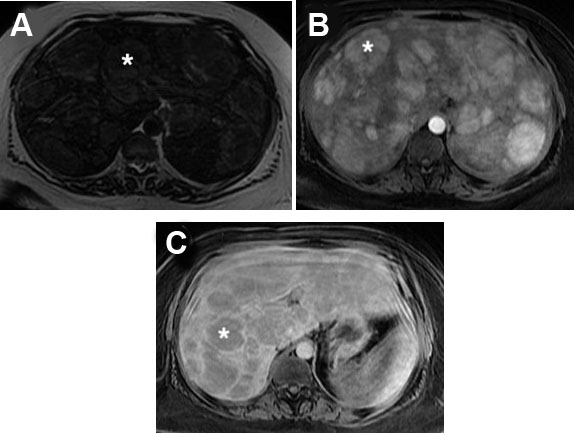
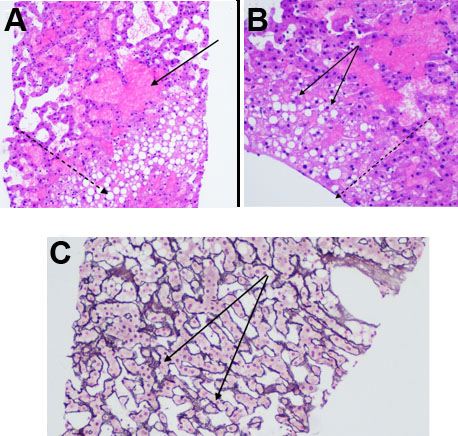
A 33-year-old black female with a past medical history of seizures, hypertension, type-2 diabetes, cognitive impairment, and neuropathy resulting in complete loss of ambulation was referred to our institution for radiologic evaluation following an abnormal liver function test (alkaline phosphatase (ALP) 1193; alanine transaminase/aspartate transaminase (ALT/AST) were normal at 16/16 U/L), microcytic anemia, and a vitamin B12 deficiency. No alcohol, drug, tobacco use, or family history of liver disease were noted. On physical exam, jaundice and abdominal pain were absent; chronic fatigue had been present for several months. Abdominal ultrasound (Figure 1A and Figure 1B) revealed hepatomegaly that exceeded 23 cm, with multiple heterogenous masses in both lobes of the liver hyperechoic to the surrounding parenchyma; further evaluation with contrast-enhanced CT (Figure 2A, Figure 2B, Figure 2C) revealed enhancement of approximately 100 lesions throughout both liver lobes, the largest measuring 5–6 cm. The liver parenchyma had a uniform density without pathologic enhancement and a normal gallbladder without evidence of biliary dilation. Magnetic resonance imaging (MRI) (Figure 3A, Figure 3B, Figure 3C) shows numerous T2 hyperintense hepatic lesions, which enhanced during the arterial phase with intravenous (IV) gadolinium. To rule out a hyper-vascular primary malignancy or vascular metastasis, a CT-guided percutaneous biopsy of the liver was performed. Pathology identified mild macro-vesicular steatosis and marked sinusoidal dilation (Figure 4A, Figure 4B, Figure 4C), consistent with peliosis hepatis and mild macro-vesicular steatosis of the liver. Occult bleeding of the upper and lower gastrointestinal tract (GI) tract was ruled out; the anemia was treated with Vitamin B12 injections and IV iron transfusions. After two years of follow-up, the anemia persisted; testing revealed alfa-thalassemia minor. Radiographic follow-ups noted stable lesions; the patient has remained asymptomatic three-years post-diagnosis. Interestingly, however, the patient’s lab values have continued to show elevated ALP levels that vary between 1200 and 1700 IU/L, while the ALT/AST values have remained normal.
Discussion
Peliosis hepatis has been associated with certain medications, infections, hematologic conditions, and transplantation [1],[4],[7],[13],[26],[27],[28],[29] (Table 1 and Table 2). Symptomatic PH (n=49; 78%) presents with abdominal pain (40%; n=25), GI symptoms/weight loss (21%; n=13), and abdominal fullness/hepatomegaly/mass (19%; n=12; Table 1) [1],[3],[4],[6],[30]. Fatigue, as in our patient, was reported rarely (8%; n=5). Of those that reported outcomes (n=48), mortality was 27% (n=13).
Abnormal lab values (64%; n=40), specifically, elevated liver function tests (38%; n=24), were common [1],[3],[9],[31]: ALP (52.6%; n=10), ALT (47.4%; n=9), gamma-glutamyl transferase (GGT) (42.1%; n=8) and AST (31.6%; n=6). Interestingly, the elevated ALP in our patient (1100–1700 IU/L) is the highest reported (mean 391 IU/L; Table 1), and the consistently normal AST/ALT is the first reported (Table 1) [1],[6],[12]. Anemia (35%; n=22), thrombocytopenia (19%; n=12), leukocytosis (11%; n=7), and hyperbilirubinemia (10%; n=6) were also reported. For patients with anemia, as with our patient, colonoscopy (7.9%; n=5) and upper GI endoscopy (EGD) (15.9%; n=10) were completed (Table 1).
A majority of asymptomatic patients (22%; n=14; Table 1) were diagnosed incidentally by imaging (71%; n=10), with variable findings within lesional tissue and the concomitant presence of hepatic steatosis [9],[16],[17],[26],[32]. Vascular tumors, hepatic adenomas, hepatocellular carcinoma, hepatic abscesses and other cystic liver diseases remained in the differential [4],[6],[12],[13],[18],[27], with normal magnetic resonance pancreatography (MRCP) (6.3%; n=4) and fluorodeoxyglucose-positron emission tomography (FDG-PET) (11.1%; n=7) ruling out malignant processes [1],[4],[12].
Diagnostic imaging is essential in evaluating PH due to biopsy/surgery bleeding risk. Initial ultrasound (55.6%; n=35) identified homogenous/hypoechoic lesions with hepatic steatosis or hyperechoic lesions with normal hepatic parenchyma [16],[26], as noted in our patient (Figure 1). Doppler studies can identify blood flow and thrombi or portal obstruction [6],[16],[26]. Contrast-enhanced CT (54.0%; n=34), the preferred imaging modality [4],[16],[18],[26],[32],[33],[34], differentiates hypoattenuating peliotic lesions versus normal parenchyma [16],[18],[32],[33],[34],[35]; characteristically, early central enhancement during the arterial phase with centrally accumulating contrast in a “target sign” that diffuses to the periphery during the venous phase is noted in PH [16],[18],[26],[33],[34],[35]. With an active hemorrhage, hyperattenuating contrast material may accumulate or leak during the late phase [4],[16],[18],[26],[32],[33],[34]. The initial CT in our report revealed multiple enhancing lesions throughout the liver parenchyma, the largest 5–6 cm (Figure 2).
Magnetic resonance imaging (MRI) was a commonly utilized imaging modality (40.0%; n=25) with MRI T1-weighted images appearing hypointense with an enhancement that progresses centrifugally; cavities display a rim of enhancement with hematoma [17],[18],[26],[35]. T2-weighted MRI images appear hyperintense to the parenchyma with multiple foci of high signal, consistent with what was seen in our patient using Gadolinium contrast enhancement (Figure 3) [16],[17],[18],[26].
Diagnostic angiography identifies contrast accumulation during the late arterial phase and persistent enhancement during the venous phase with multiple vascular nodules [4],[6],[8],[20],[36]; angiography also allows embolization to commence [36],[37],[38]. Interestingly, reported angiography noted a right-sided predominance of PH (70.6%) with a greater incidence of hemorrhage, most often from the right hepatic artery (62.5%).
Definitive diagnosis of PH currently relies on histologic identification of blood-filled cystic dilated sinusoids throughout the liver parenchyma with evidence of rupture of reticulin fibers or necrosis of hepatocytes and macrovesicular steatosis (Figure 4) [4],[6],[10],[12],[15],[27],[30],[39]; however, due to the risk of hemorrhage (26.3%; n=12) or death (41.7%; n=5), biopsy is only warranted when imaging reveals a concern for malignancy [6],[12],[25],[39]. Thus, monitoring of PH through serial imaging and liver function testing [1],[11],[40],[41],[42],[43], may be appropriate.
Prophylactic surgical resection of affected tissue may be considered in patients with worsening cavitary lesions to prevent spontaneous cyst rupture and hemorrhage (n=7) [4],[8],[19],[20],[21],[22], but this can be complicated by hemorrhage and death (n=1; Table 1) [44]. Emergent surgical management or embolization is reserved for those with evidence of acute hemorrhage (36.5%; n=23) [6],[8],[36],[40]: surgical resection (56.5%; n=13), embolization (39.1%; n=9), and embolization followed by surgical resection (n=1). Complications and mortality were noted following resection (23%; n=3 and 30%; n=1, respectively) and embolization (44.4%; n=4 and 50%; n=2, respectively), but reports are few.
Currently, there is no specific treatment for PH, and lesions have been reported to spontaneously resolve without clinical consequence (7.9%; n=5) [4],[13],[23],[29]; specifically, drug/toxicity-related cases (7.9%; n=5) often resolve with withdrawal of the offending drug/toxin with normalization of lab values. Peliosis hepatis lesions due to Bartonella may regress with antibiotics, such as Doxycycline or Erythromycin; however, bacillary peliosis cases were not identified in the literature [45]. Typically, however, progression to portal hypertension and liver failure occurs when PH remains untreated (22%; n=14) [1],[4],[6],[13],[29] with ascites (58.3%; n=7), portal hypertension (25%; n=3), liver failure (25%; n=3), and encephalopathy (8.3%; n=1) reported; mortality due to long-term complications of hepatic failure was also noted (16.7%; n=2).
While there is no consensus for routine monitoring in asymptomatic patients or those who refuse surgery, serial imaging (55.6%; n=5) and liver function tests have been suggested (14.3%; n=9) [37]; intervals of three months (n=3), six months (n=2), and one year (n=1) have been reported. Of these, 44.4% had stable disease, regression/normal labs were noted in 33.3% and 22.2% died from progressive hepatic failure.
Conclusion
The dilatation of hepatic sinusoids in patients with PH poses a risk for morbidity and mortality due to spontaneous or surgically induced rupture and hemorrhage, necessitating early and accurate diagnosis. Unfortunately, most patients are asymptomatic, and, when diagnostic imaging such as sonography, CT, MRI, and angiography suspects PH, a biopsy is the only means by which to obtain a definitive pathologic diagnosis. However, as tissue procurement risks hemorrhage, and there is no consensus regarding standard treatment, patients should be evaluated initially for drug/toxicity-related PH for which regression may occur with removal of the offending agent. If there is no concern for malignancy, and for asymptomatic patients and those without indications of advanced liver disease, serial imaging and liver function testing may be appropriate management, with resection and embolization reserved for those with evidence of acute hemorrhage.
REFERENCES
1.
Crocetti D, Palmieri A, Pedullà G, Pasta V, D’Orazi V, Grazi GL. Peliosis hepatis: Personal experience and literature review. World J Gastroenterol 2015;21(46):13188–94. [CrossRef]
[Pubmed]

2.
Siddiqi I, Gupta N. Peliosis Hepatis. 2022 Jun, 21. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
[Pubmed]

3.
Zak FG. Peliosis hepatis. Am J Pathol 1950;26(1):1–15.
[Pubmed]

4.
Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int 2005;149(1):25–33. [CrossRef]
[Pubmed]

5.
Kleger A, Bommer M, Kunze M, et al. First reported case of disease: Peliosis hepatis as cardinal symptom of Hodgkin’s lymphoma. Oncologist 2009;14(11):1088–94. [CrossRef]
[Pubmed]

6.
Elsayes KM, Shaaban AM, Rothan SM, et al. A comprehensive approach to hepatic vascular disease. Radiographics 2017;37(3):813–36. [CrossRef]
[Pubmed]

7.
Modlinski R, Fields KB. The effect of anabolic steroids on the gastrointestinal system, kidneys, and adrenal glands. Curr Sports Med Rep 2006;5(2):104–9. [CrossRef]
[Pubmed]

8.
Srinivasa S, Lee WG, Aldameh A, Koea JB. Spontaneous hepatic haemorrhage: A review of pathogenesis, aetiology and treatment. HPB (Oxford) 2015;17(10):872–80. [CrossRef]
[Pubmed]

9.
Maves CK, Caron KH, Bisset GS 3rd, Agarwal R. Splenic and hepatic peliosis: MR findings. AJR Am J Roentgenol 1992;158(1):75–6. [CrossRef]
[Pubmed]

10.
Yanoff M, Rawson AJ. Peliosis hepatis. An anatomic study with demonstration of two varieties. Arch Pathol 1964;77:159–65.
[Pubmed]

11.
Radin DR, Kanel GC. Peliosis hepatis in a patient with human immunodeficiency virus infection. AJR Am J Roentgenol 1991;156(1):91–2. [CrossRef]
[Pubmed]

12.
13.
Zafrani ES, von Pinaudeau Y, Dhumeaux D. Drug-induced vascular lesions of the liver. Arch Intern Med 1983;143(3):495–502.
[Pubmed]

14.
Steinke K, Terraciano L, Wiesner W. Unusual cross-sectional imaging findings in hepatic peliosis. Eur Radiol 2003;13(8):1916–9. [CrossRef]
[Pubmed]

15.
Savastano S, San Bortolo O, Velo E, Rettore C, Altavilla G. Pseudotumoral appearance of peliosis hepatis. AJR Am J Roentgenol 2005;185(2):558–9. [CrossRef]
[Pubmed]

16.
Iannaccone R, Federle MP, Brancatelli G, et al. Peliosis hepatis: Spectrum of imaging findings. AJR Am J Roentgenol 2006;187(1):W43–52. [CrossRef]
[Pubmed]

17.
Battal B, Kocaoglu M, Atay AA, Bulakbasi N. Multifocal peliosis hepatis: MR and diffusion-weighted MR-imaging findings of an atypical case. Ups J Med Sci 2010;115(2):153–6. [CrossRef]
[Pubmed]

18.
19.
Biswas S, Gogna S, Patel P. A fatal case of intra-abdominal hemorrhage following diagnostic blind percutaneous liver biopsy in a patient with peliosis hepatis. Gastroenterol Res 2017;10(5):318–21. [CrossRef]
[Pubmed]

20.
Downes RO, Cambridge CL, Diggiss C, Iferenta J, Sharma M. A case of intra-abdominal hemorrhage secondary to peliosis hepatis. Int J Surg Case Rep 2015;7C:47–50. [CrossRef]
[Pubmed]

21.
Choi SK, Jin JS, Cho SG, et al. Spontaneous liver rupture in a patient with peliosis hepatis: A case report. World J Gastroenterol 2009;15(43):5493–7. [CrossRef]
[Pubmed]

22.
Khadilkar UN, Prabhu S, Sharma D. Peliosis hepatis presenting as hemoperitoneum. Indian J Med Sci 2008;62(6):236–7. [CrossRef]
[Pubmed]

23.
Elsing C, Placke J, Herrmann T. Alcohol binging causes peliosis hepatis during azathioprine therapy in Crohn’s disease. World J Gastroenterol 2007;13(34):4646–8. [CrossRef]
[Pubmed]

24.
Cohen GS, Ball DS, Boyd-Kranis R, Gembala RB, Wurzel J. Peliosis hepatis mimicking hepatic abscess: Fatal outcome following percutaneous drainage. J Vasc Interv Radiol 1994;5(4):643–5. [CrossRef]
[Pubmed]

25.
Hong GS, Kim KW, An J, Shim JH, Kim J, Yu ES. Focal type of peliosis hepatis. Clin Mol Hepatol 2015;21(4):398–401. [CrossRef]
[Pubmed]

26.
Yang DM, Jung DH, Park CH, Kim JE, Choi SJ. Imaging findings of hepatic sinusoidal dilatation. AJR Am J Roentgenol 2004;183(4):1075–7. [CrossRef]
[Pubmed]

27.
Dai YN, Ren ZZ, Song WY, et al. Peliosis hepatis: 2 case reports of a rare liver disorder and its differential diagnosis. Medicine (Baltimore) 2017;96(13):e6471. [CrossRef]
[Pubmed]

28.
Cavalcanti R, Pol S, Carnot F, et al. Impact and evolution of peliosis hepatis in renal transplant recipients. Transplantation 1994;58(3):315–6.
[Pubmed]

29.
Biswas S, Rolain JM. Bartonella infection: Treatment and drug resistance. Future Microbiol 2010;5(11):1719–31. [CrossRef]
[Pubmed]

30.
Berzigotti A, Magalotti D, Zappoli P, Rossi C, Callea F, Zoli M. Peliosis hepatis as an early histological finding in idiopathic portal hypertension: A case report. World J Gastroenterol 2006;12(22):3612–5. [CrossRef]
[Pubmed]

31.
Hiorns MP, Rossi UG, Roebuck DJ. Peliosis hepatis causing inferior vena cava compression in a 3-year-old child. Pediatr Radiol 2005;35(2):209–11. [CrossRef]
[Pubmed]

32.
Ferrozzi F, Tognini G, Zuccoli G, Cademartiri F, Pavone P. Peliosis hepatis with pseudotumoral and hemorrhagic evolution: CT and MR findings. Abdom Imaging 2001;26(2):197–9. [CrossRef]
[Pubmed]

33.
Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics 2008;28(7):1967–82. [CrossRef]
[Pubmed]

34.
Kleinig P, Davies RP, Maddern G, Kew J. Peliosis hepatis: Central “fast surge” ultrasound enhancement and multislice CT appearances. Clin Radiol 2003;58(12):995–8. [CrossRef]
[Pubmed]

35.
Gouya H, Vignaux O, Legmann P, de Pigneux G, Bonnin A. Peliosis hepatis: Triphasic helical CT and dynamic MRI findings. Abdom Imaging. 2001;26(5):507–9. [CrossRef]
[Pubmed]

36.
Oriordan K, Blei A, Vogelzang R, Nemcek A, Abecassis M. Peliosis hepatis with intrahepatic hemorrhage: Successful embolization of the hepatic artery. HPB Surg 2000;11(5):353–8. [CrossRef]
[Pubmed]

37.
Bleibel W, Curry MP. Peliosis hepatis. UpToDate 2021. [Available at: https://www.uptodate.com/contents/peliosis-hepatis?search=peliosis%20hepatis&source=search_result&selectedTitle=1~34&usage_type=default&display_rank=1#H52050050]

38.
Mohr AM, Lavery RF, Barone A, et al. Angiographic embolization for liver injuries: Low mortality, high morbidity. J Trauma 2003;55(6):1077–81. [CrossRef]
[Pubmed]

39.
Vignaux O, Legmann P, de Pinieux G, et al. Hemorrhagic necrosis due to peliosis hepatis: Imaging findings and pathological correlation. Eur Radiol 1999;9(3):454–6. [CrossRef]
[Pubmed]

40.
Pan W, Hong HJ, Chen YL, Han SH, Zheng CY. Surgical treatment of a patient with peliosis hepatis: A case report. World J Gastroenterol 2013;19(16):2578–82. [CrossRef]
[Pubmed]

41.
Demyashkin G, Zatsepina M. Peliosis hepatis complicated by portal hypertension following renal transplantation. World J Gastroenterol 2020;26(34):5220–2. [CrossRef]
[Pubmed]

42.
Hidaka H, Ohbu M, Nakazawa T, Matsumoto T, Shibuya A, Koizumi W. Peliosis hepatis disseminated rapidly throughout the liver in a patient with prostate cancer: A case report. J Med Case Rep 2015;9:194. [CrossRef]
[Pubmed]

43.
Zhang Y, Tong A, Maddineni S. The glass chameleon—Peliosis hepatis: A diagnostic challenge for the radiologist and a danger for the interventionalist. J Vasc Interv Radiol 2016;27(3):S244. [CrossRef]

44.
Atila K, Coker A, Uçar D, et al. A rare clinical entity misdiagnosed as a tumor: Peliosis hepatis. Ulus Travma Acil Cerrahi Derg 2007;13(2):149–53.
[Pubmed]

45.
Luciani L, El Baroudi Y, Prudent E, Raoult D, Fournier PE. Bartonella infections diagnosed in the French reference center, 2014–2019, and focus on infections in the immunocompromised. Eur J Clin Microbiol Infect Dis 2021;40(11):2407–10. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Alex Teshon - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Crystal Walker - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
David P Schammel - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christine Marie-Gilligan Schammel - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
A Michael Devane - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Alex Teshon et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.