|
Case Report
Unveiling the enigma of spontaneous regression in hepatocellular carcinoma: A case report from a tertiary care center in South India
1 Ida B. Scudder Cancer Centre, Department of Radiation Oncology, Christian Medical College, Vellore, Tamil Nadu, India
2 Department of Radiodiagnosis, Christian Medical College, Vellore, Tamil Nadu, India
3 Department of Hepatobiliary and Pancreatic Surgery, Christian Medical College, Vellore, Tamil Nadu, India
4 Department of Pathology, Christian Medical College, Vellore, Tamil Nadu, India
5 Department of Hepatology, Christian Medical College, Vellore, Tamil Nadu, India
Address correspondence to:
Thomas Samuel Ram
Ida B. Scudder Cancer Centre, Department of Radiation Oncology Unit 1, Christian Medical College, Vellore, Tamil Nadu,
India
Message to Corresponding Author
Article ID: 100105Z04RK2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Benny RK, Tony V, Thanikaivelu S, Raju RS, Rijo Isaac NP, Barman B, Kiruba A, Thomas RS, John NO, Sathyamurthy A, Ramireddy JK, Ram TS. Unveiling the enigma of spontaneous regression in hepatocellular carcinoma: A case report from a tertiary care center in South India. Int J Hepatobiliary Pancreat Dis 2024;14(2):15–26.ABSTRACT
Introduction: Spontaneous regression in hepatocellular carcinoma (HCC) is an enigma as it is a very rare phenomenon, multiple possible hypotheses were described to support this enigma.
Case Report: A 61-year-old man known Type 2 diabetes mellitus and hypertension was evaluated for complaints of unexplained weight loss (40 kg loss in eight months), loss of appetite, along with generalized weakness of three months duration. He underwent computed tomography (CT) scan abdomen that showed arterial phase hyper-enhancing lesion (white short arrow) in left lobe/segment V of liver with washout. His alpha-fetoprotein (AFP) was 12263 IU. He was advised transarterial radioembolization (TARE) and systemic therapy. He did not undergo any treatment due to logistical issues. After three months he underwent a repeat CT scan, which showed decrease in the size of the heterogeneously hypodense space-occupying lesion (SOL) with wall irregularity involving liver segments II, III, IV, and V. His AFP level had fallen to 600 IU. He underwent a diagnostic laparoscopy, intraoperative ultrasound scan, frozen section (a rapid intraoperative histopathological diagnosis) proceeds left hepatectomy (including distal middle hepatic vein) and excision of 2 lesions in the caudate lobe and cholecystectomy under general anesthesia. The left hepatectomy specimen showed a scanty viable tumor (~5%) consistent with moderately differentiated hepatocellular carcinoma and with secondary changes (~95%), including extensive necrosis, xanthogranulomatous inflammation, and hemorrhage. He was followed up for three years with serial CT scan and was found to be disease free with 3 years AFP value of 1.32 IU.
Conclusion: We conclude that partial spontaneous resolution of hepatocellular carcinoma (HCC) is rare but a possible phenomenon with multiple mechanisms explaining the enigma and it presents an opportunity for further research. The collection and thorough analysis of clinical data obtained from patients who have experienced spontaneous resolution of HCC will help understand this mysterious phenomenon. It could also lead to the development of new treatment strategies for HCC based on the possible hypothesis.
Keywords: AFP, Biopsy proven, Hepatocellular carcinoma, Spontaneous resolution
Introduction
Hepatocellular carcinoma (HCC) is recognized to be one of the common types of primary liver cancer, with the highest prevalence globally, imposing a substantial burden on public health [1]. In 2020, HCC was responsible for approximately 906,000 new cases and 830,000 deaths, making it the sixth most prevalent cancer worldwide. Hepatocellular carcinoma is the third leading cause of cancer-related deaths globally, with incidence rates rising steadily over the past decades [2]. The North American Association of Central Cancer reported that an estimated 41,210 new HCC cases were diagnosed in the United States in 2023, with incidence rates tripling over the past 40 years [3]. Chronic infection due to hepatitis B or C viruses remains a major risk factor globally, accounting for a considerable proportion of HCC cases [4]. Other risk factors include cirrhosis, aflatoxin exposure, excessive alcohol consumption, diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), and certain genetic disorders like hemochromatosis and alpha-1 antitrypsin deficiency [5],[6].
Patients with HCC may remain asymptomatic in the initial stages, leading to delayed diagnosis and poorer outcomes. Common clinical manifestations include abdominal pain, weight loss, jaundice, and hepatomegaly [7]. Diagnostic modalities include imaging studies such as ultrasound, CT scan (triple phase), magnetic resonance imaging (MRI) with contrast, and serum biomarkers like alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP).
According to the guidelines of American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL), a liver lesion which is larger than 2 cm with the vascular pattern typical on contrast-enhanced CT (CECT) or contrast-enhanced MRI can be considered HCC without biopsy [8],[9]. Lesions in the liver which measures between 1 and 2 cm, HCC diagnosis is established when typical vascular pattern is seen on both the diagnostic imaging. Otherwise, these atypical lesions should not be treated as HCC and a histological evidence is compulsory because of a rate of false positives as high as 20% [10],[11].
Liver biopsy is not routine practice for diagnosis of HCC. However, if the imaging features are not conclusive for HCC, histopathological examination through liver biopsy may be required [12].
Managing HCC depends on numerous factors, including tumor stage, liver function, and the patient’s overall health. Treatment options encompass surgical resection, liver transplantation, locoregional therapies (e.g., radiofrequency ablation, transarterial chemoembolization [TACE], transarterial radioembolization [TARE], stereotactic body radiation therapy [SBRT]), systemic therapies (e.g., sorafenib and Lenvatinib). Early-stage HCC is associated with a better prognosis and higher chances of curative treatment, whereas advanced-stage disease carries a poorer prognosis with limited therapeutic options.
Spontaneous regression of HCC was first defined by Everson and Cole in year 1959 as partial or complete disappearance of the malignant tumors when there was no treatment taken by the patient [13] and it is a well-established phenomenon in certain malignancies like renal cell carcinoma, neuroblastoma, and choriocarcinoma [14]. Spontaneous resolution of HCC has rarely been observed and was first described in literature by Johnson and colleagues in 1972 [15]. We describe our observation of spontaneous, near-complete resolution of HCC in a 61-year-old gentleman.
Case Report
A 61-year-old man was evaluated for complaints of unexplained weight loss (40 kg loss in eight months), loss of appetite, and generalized weakness of three months duration.
He had no history of alcohol consumption or smoking.
Past history
He consistently took medications for his Type 2 diabetes mellitus on metformin and glimepride and hypertension on cilnidipine, chlorthalidone, mirabegron, telmisartan, metoprolol, asprin, and atorvastatin. He had undergone a surgery for hydrocele in 2002, a transurethral resection of the prostate (TURP) in 2014 for Benign prostatic hyperplasia and a right knee replacement in 2016.
At diagnosis
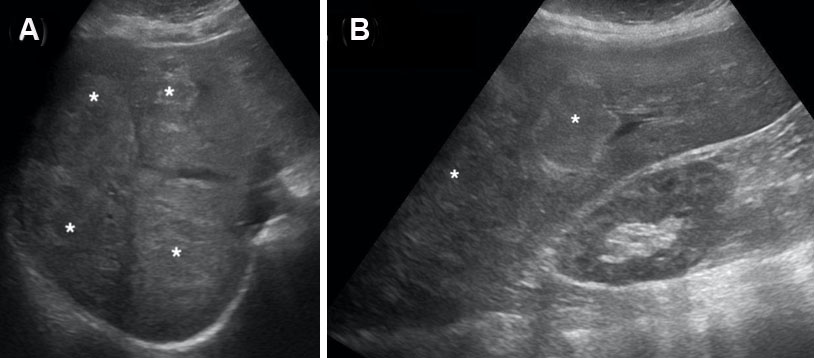
Computed tomography (triphasic) of the abdomen and pelvis showed a 20×15×12 cm sized heterogeneously enhancing space occupying lesion (SOL) with wall irregularity involving both lobes of the liver, predominantly segments II, III, IV, and V. There was loss of fat planes with the lesser curvature of the stomach. The background liver showed nodular outline with volume redistribution suggesting chronic liver parenchymal disease. The post-contrast study (arterial phase) showed irregular heterogeneous areas of enhancement with central non-enhancing necrotic regions. The venous phase showed a complete washout of contrast from the SOL, and the findings suggested a neoplastic mass with no portal vein thrombosis. The lesion was liver imaging reporting and data system (LIRADS) grade 5, possibly hepatocellular carcinoma (Figure 1A, Figure 1B, Figure 1C, Figure 1D).
His blood investigations showed raised alpha-fetoprotein (AFP-12263 ng/mL). His clinical and radiological details were discussed in a multi-disciplinary tumor board meeting, and he was clinicoradiologically diagnosed to have hepatocellular carcinoma [16] and was advised transarterial radioembolization (TARE) and systemic therapy. He did not undergo any treatment due to logistic issues.
After three months
At his next review, three months later, a repeat CT scan was done and it showed a 12.0×7.0×6.0 cm sized heterogeneously hypodense SOL with wall irregularity involving liver segment II, III, IV, and V (Figure 2 and Figure 3). His AFP level had fallen to 600 IU.
Liver stiffness measurement with ARFI showed F3/F4 fibrosis (median 1.8 m/s). In view of long-standing history of diabetes mellitus and hypertension, the etiology for the underlying liver disease was considered likely to be non-alcoholic fatty liver disease (NAFLD) related after excluding hepatotropic viruses, vascular and autoimmune etiologies. There was no evidence of portal hypertension (normal sized spleen, normal portal vein diameter, normal platelet count, no portosystemic collaterals) and no tumor thrombosis in portal vein.
During this three months of duration, he declined the use of any kind of alternative medications but acknowledged that he had reduced appetite and ate less.
After five months
Magnetic resonance imaging done at his following review, five months after diagnosis, showed a complex mixed signal intensity lesion with T2 hypointense areas in the left lobe of the liver, measuring approximately 11×7×5 cm, with multiple adjacent small hyperintense foci (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9).
His AFP was further reduced to 392 IU, and his liver function test was within normal limits.
His case was rediscussed at the hepato-pancreatic-biliary multidisciplinary tumor board, and it was considered potentially resectable after assessment of liver function with indocyanine green test (ICG). His gastroscopy was negative for varices and indocyanine green clearance test R 15 value was of 12.1%. The volume of the right lobe was 1400 cc. He underwent surgical excision of the lesion.
Surgery and intra-op findings
He underwent a diagnostic laparoscopy, scan, and frozen section (a rapid intraoperative histopathological diagnosis). Following these procedures, he proceeded with a left hepatectomy (including distal middle hepatic vein), excision of two lesions in the caudate lobe, and cholecystectomy under general anesthesia. His intra-op diagnostic laparoscopy showed a moderately fatty liver (hepatic steatosis) with a nodular surface. There are no apparent lesions on the peritoneum or liver surface. There was a nodular and hard ill-defined lesion—at least 8 × 7 cm in segment II and the superior aspect of segment III, extending to segment IVA up to the middle hepatic vein. There were four discrete lesions in the right lobe of the liver, which were sent for frozen section and were reported to be benign. There was a small lesion on the right side of the caudate lobe on the inferior aspect and a 1.5 × 2 cm lesion on the left lateral aspect of the caudate lobe, which was also excised. No other lesions were seen on the intraoperative ultrasound scan, and no enlarged nodes were found in the hepatoduodenal ligament.
Surgical histopathology report
His frozen and paraffin sections from segment 5, segment 6 anterior, segment 6 posterior, and segment 7 liver lesions were reported as bile duct adenomas and adenofibromas.
The left hepatectomy specimen showed a scanty viable tumor (~5%) consistent with moderately differentiated hepatocellular carcinoma and with secondary changes (~95%), including extensive necrosis, xanthogranulomatous inflammation, and haemorrhage.
Tumor dimension of viable focus was 0.7 cm. Vascular invasion was not present (Figure 10, Figure 11, Figure 12).
Immunohistochemistry (IHC): The tumor nodule showed diffuse positivity for Glypican3. Smooth muscle actin (SMA) highlighted the background myofibroblasts. Immunohistochemistry for MelanA, HMB45, and ALK1 was negative (Figure 13).
Background liver showed edema, mild to moderate portal inflammation, vascular ectasia, bile duct proliferation, and portal and periportal focal bridging fibrosis. The nearest necrotic foci lay 0.5 cm from the liver resection margin—hepatic vein resection margin was free of tumor.
Gall bladder showed chronic cholecystitis with haemorrhage. He was surgically staged as pT1a disease. His postoperative period was uneventful. Postoperative CT of abdomen/pelvis done two weeks post-surgery showed postoperative changes with no evidence of residual/recurrent disease (Figure 14A and Figure 14B).
He was discussed in a multidisciplinary tumor board conference and was kept under active surveillance.
He was followed up for three years post-surgery with annual cross-sectional imaging and serum AFP, and he was found to be disease free.
First year post-diagnosis
He was reviewed after one year post-diagnosis and he was asymptomatic clinically. He underwent a CT scan which showed postoperative changes in the form of surgical clips at the anastomotic margin and mild fat stranding in the adjacent omentum. No focal lesion was seen (Figure 15). His AFP was 1.91.
Second year post-diagnosis
His repeat CT showed postoperative changes in the form of surgical clips at the anastomotic margin. No focal lesion was seen (Figure 16). His AFP was 1.9.
Third year post-diagnosis
His repeat CT showed postoperative changes in the form of surgical clips at the anastomotic margin. No focal lesion was seen (Figure 17). His AFP was 1.32.
Discussion
Advanced HCC typically exhibits a high morbidity rate with restricted therapeutic options and generally poor prognoses [17]. Spontaneous regression in HCC is an exceptionally rare occurrence, estimated at approximately one in 140,000 cases, with few documented instances in medical literature [18].
Here, we report an unusual case of HCC with a clinical course of spontaneous, near-complete tumor regression.
A review of the literature showed that the first case report of spontaneous resolution of HCC was in 1972 by Johnson and colleagues. A 3-year-old girl had been diagnosed with biopsy-proven HCC while on chronic androgen-anabolic steroid treatment for aplastic anemia. One year after her diagnosis, she received treatment for Staphylococcus aureus septicaemia, during which her androgen-anabolic steroid treatment was withheld, during assessment her imaging demonstrated near-complete resolution of her HCC, which suggests that an androgenic environment favors development of hepatocellular carcinoma [15].
Toyoda et al. estimated spontaneous resolution from 10 randomized controlled trials observed in 1640 patients with diagnosed HCC. Based on the review, the incidence of regression in the control groups was calculated as 0.4% [19].
Interestingly, there have been few case reports of spontaneous (complete) regression of HCC [17]. Most cases showed partial regression, while a few demonstrated complete regression. There is no correlation between the underlying etiology of liver disease and the spontaneous resolution of HCC. The index case had no cirrhosis, and laboratory findings and radiological studies did not show evidence of chronic hepatitis or cirrhosis. Spontaneous resolution of HCC with distant metastasis to the lungs [20], chest wall [21], skull [22], peritoneum, and spleen [23] has been reported. Tumor regression of assorted sizes has been described in the literature, and the outcome in most cases has been favorable with few recurrences. Survival was prolonged when surgical resections were implicated [24].
Although several factors, such as reduction in blood supply, inflammation, and immunological factors, have been suggested as the mechanism of spontaneous regression of HCC, the precise mechanism still requires further study [17],[25].
Several mechanisms to explain this unusual phenomenon have been proposed. The possible explanations have been divided into three major groups:
- Impairment of the blood supply due to arterial thrombosis,
- Immune reaction,
- Other factors:
- Abstinence from alcohol consumption,
- Use of herbal medicines,
- Systemic inflammatory reactions,
- Abscopal effect,
- Discontinuation of immunosuppressive therapy,
- Sudden enlargement of the tumor,
- Reduced blood supply to cancer cells due to the formation of a fibrous capsule,
- Administration of vitamin K,
- Stoppage of exogenous androgens
- Tumor genomic alterations and immune microenvironment.
Impairment of the blood supply due to arterial thrombosis
Arterioportal shunt which is present near the tumor alters the blood flow dynamics to the cancer cell, for the growth of the tumor blood flow is very essential. Altering of the blood flow leading to spontaneous regression phenomena is similar to commonly used procedure in the treatment of HCC which is transarterial chemoembolization (TACE). Due to alteration of the blood flow, the tumor in turn undergoes infarction which is caused by the disruption of the feeding vessels or the portal vein. This alteration of the blood flow can be caused due to various mechanisms like subintimal injury of the vessel, thrombus formation, and direct tumor invasion in to the vessel. This results in tumor necrosis which leads into the regression of HCC. Another explanation considered is coagulative necrosis produced due to interruption in the blood supply, leading to the spontaneous formation of an arterial thrombus [26],[27],[28].
Immune reaction
In one report, treatment by activated T-lymphocytes has been shown to reduce recurrence rates of HCC after surgery [29]. This finding suggests that related regression may be associated with host immune response involving cytokines such as tumor necrosis factor-alpha [30]. The immune reaction might have played a significant role because the tumor stroma of HCC is infiltrated by many CD8+ T cells. These mediate cytotoxic killing due to which the antitumor immune reaction is present in the tumor microenvironment. The immune cells, i.e., T cells, B cells, and natural killer cells, represent the host antitumor immune response [31]. Increased numbers of tumor-infiltrating lymphocyte (TILs), like activated cytotoxic T cells, correlate with better survival in malignant tumors, including HCC [32]. A large meta-analysis revealed that elevated intratumoral levels of CD8+ cells has shown to be associated with better overall survival [33]. However, there are several studies which has reported to have contradictory findings which make the the prognostic role of the TILs for survival controversial [34],[35].
Other factors
Abstinence from alcohol consumption
Storey et al. published a case report of complete regression of the HCC with possible lung metastasis by computed tomography caused by abstinence from alcohol. This was confirmed by surgery and pathology [35],[36],[37].
Use of herbal medicines
Some herbal drugs, including Actinidia valvata Dunn, Toosendanin, Curcumae Radix, and Artemisia carvifolia have been verified for antitumor activity on HCC. Saponin is extracted from root of A. valvata plant and it has been reported to have anti-HCC activity in vitro for HCC cells in cell lines BEL-7402, HepG2, PLC, SMMC-7721, MHCC-97-H, and MHCC-97-L [33]. The extract acts by restraining of the adhesion, invasion, mobility, and migration abilities of the following cells in vitro: BEL-7402 and MHCC-97-H [38]. The extract toosendanin has shown to have potent anti-HCC effects by the process of suppressing of proliferation of the cells and thereby leading to apoptosis of the cancer cells which was seen in vitro along with the cell lines of HCC SMMC-7721 and Hep3B was proved in vivo with the BALB/c mice. The mechanism of apoptosis involves the mitochondrial pathway and death receptor pathway [39]. Curcumin, which is extracted from Curcumae Radix root, has demonstrated synergistic effect when used along with bevacizumab by inhibiting the effects of the vascular endothelial growth factor (VEGF) signaling pathways, which is commonly seen as leading to HCC progression [40]. Beta-element, which is well known for the sensitizing nature and antitumor activity for the of HCC cells to chemotherapeutic agents like oxaliplatin, can also be extracted from Curcumae Radix [41]. Despite all these findings which showed benefits of use of Curcumae Radix and the changes caused by chemical reactions when these are mixed and boiled together remain an unsolved mystery in the current scientific world.
Systemic inflammatory reactions: A systemic process could be one of the inciting factors underlying the spontaneous regression. Several reports documented elevated cytokine levels, suggesting a systemic inflammatory response. Abiru et al. noted elevated IL-18 in three patients who had regression of HCC. IL-18 has also been shown to induce interferon (IFN)-gamma production by both T-cells and natural killer (NK) cells, thus potentially producing enhanced cytotoxic activity targeted at cancer cells [41],. Jozuka et al. proposed a mechanism after detecting elevated levels of inflammatory markers with NK cell activity, IFN-gamma IL-2, IL-6, and IL-12 throughout the patient’s spontaneous regression [42], [43].
While patients with cytokine elevation were known to have been associated with infections, prominent levels of the same were also seen in non-infectious patients. The report by Blondon et al. describes a patient in whom tumor rupture associated with peritoneal dissemination of cancer cells caused the activation of an immune response against the tumor antigens [24].
Abscopal effect
Abscopal regression of tumors resulting from the effect of irradiation of tissue on a remote non-irradiated tissue is also rare. Regression of the primary HCC tumor after radiation therapy was delivered to metastases [44]. Ohba et al. reported metastatic hepatocellular carcinoma with vertebral metastasis regressed after radiotherapy to thoracic vertebral bone metastasis. Serum levels of tumor necrosis factor-alpha increased after radiotherapy. They suggest that the regression of the tumor due to abscopal effect can be associated with the host immune response which involves cytokines like tumor necrosis factor-alpha [29].
Tumor necrosis factor [TNF]-alpha was elevated after radiotherapy and proposed that tumor lysis may have led to the activation of a host immune response directed at tumor antigens [45].
Discontinuation of immunosuppressive therapy
Kumar et al. described two patients who developed HCC while receiving immunosuppressive therapy for Crohn’s disease and rheumatoid arthritis. Both patients showed measurable tumor regression, a-fetoprotein (AFP) decline, and prolonged survival after withdrawal from immunosuppressive treatment [45].
Sudden enlargement of the tumor
A few hypotheses are proposed in which either or both mechanisms may be in play. Probable causes of tumor ischemia include arterial thrombosis or occlusion, rapid tumor expansion that outpaces neovascularization, and the development of arterioportal shunts. Immunologic mechanisms involve immune checkpoints that are pathways required to maintain self-tolerance. Wei et al. postulated that the sudden increase in the size of the lesion facilitated the decrease in the size of HCC [46].
Reduced blood supply to cancer cell due to the formation of a fibrous capsule
Ota et al. presented two rare cases of spontaneous necrosis of HCC confirmed by surgical resection and pathological evaluation. It was postulated that there was a disturbance in the blood supply because of a thick fibrous capsule. Spontaneous necrosis was an atypical finding for HCC. However, a vascular spot in the hypovascular area during the vascular phase and a complete defect during the Kupffer phase may be necessary. Contrast-enhanced computed tomography (CECT) is a vital modality to diagnose spontaneous necrosis [47],[48].
Administration of vitamin K
Nouso et al. reported an elderly patient who was not fit for any oncological treatment received vitamin K, which was reported in literature known to suppress the growth of the HCC cells in vitro. After starting vitamin K in three months, there are case reports which have shown that the tumor markers were normalized, and the HCC size seen on cross-sectional imaging showed a markedly regression, with the imaging showing no enhancement in the early arterial phase on CT scan. They hypothesized that the regression seen in the HCC tumor size during the administration of vitamin K could be cause for the same.
Vitamin K inhibits the growth of many cancer cell lines, including HCC. The mechanisms of the anticancer action of vitamin K have yet to be precisely understood. Still, several candidates have been proposed, including the induction of apoptosis, differentiation, and cell cycle inhibition [49]. New research which has been published has shown the anticancer properties of vitamin K could occur at the level of the enzymes tyrosine kinases and phosphatase, there by modulating the various transcription factors involved in the tumor growth. In addition to the in vitro analysis, several case reports described the usefulness of vitamin K for the treatment of human cancers, including myelodysplastic syndrome (MDS) [49],[50]. In a clinical trial of vitamin K for MDS and post-MDS acute myeloid leukemia, hematological improvement is reported in four of nine patients [51]. Recently, Nouso et al. demonstrated that the development of PV tumor thrombus is inhibited, and patient survival is improved in patients with HCC after administering vitamin K [52]. These reports suggest that vitamin K has anticancer properties that can be used to treat HCC.
Stoppage of exogenous androgens
Johnson et al. reported four patients with aplastic anemia developed hepatocellular carcinoma after long-term therapy with androgenic-anabolic steroids. In one of them, there was clinical evidence of tumor regression when the androgen was withdrawn. Evidence based on epidemiological, clinical, and experimental observations suggests that an androgenic environment favors the development of HCC. With the widespread clinical usage of androgenic-anabolic steroids, it is recommended that further study to elucidate the precise relationship between androgens and hepatocellular carcinoma is needed [53].
McCaughan et al. reported two patients with androgen-induced liver tumors. One underwent liver resection. His surgical histopathology was reported as HCC. The other patient was not treated with surgery. He was on treatment with androgen therapy for paroxysmal nocturnal hemoglobinuria and hypopituitarism. After the stoppage of androgen therapy in both the patient, there was no evidence of residual tumor seen at follow-up at 10 and 14 years after diagnosis, respectively, inferring that stoppage of androgen deprivation therapy (ADT) showed spontaneous resolution in the patient who did not underwent surgery [54].
Tumor genomic alterations and immune microenvironment
In a case report by Franses et al., the authors attributed the spontaneous resolution of HCC to a direct mechanism involving immune recognition and clearance of a tumor with high tumor mutational burden (TMB), though the exact stimulus for this antitumor activity remains unclear [55]. The immune reactivity that is caused by the high TMB can lead to increased peptide neoantigen presence in a HCC cell with highly mutated genome. Tumor mutational burden can be considered as an immunotherapy biomarker [56] The prevalence of high TMB in HCC is < 1% [57], when compared to other solid tumor it is very high, so it can be considered as one of the reasons for spontaneous resolution of HCC. The presence of DAXX gene in the HCC tumor genome can also be considered for the spontaneous resolution of HCC mechanism, although the significance of the same is not clear [55].
Conclusion
We conclude that partial spontaneous resolution of hepatocellular carcinoma (HCC) is rare but a possible phenomenon with multiple mechanisms explaining the enigma and presents an opportunity for further research. The collection and thorough analysis of clinical data obtained from patients who have experienced spontaneous resolution of HCC will help understand this mysterious phenomenon. It could also lead to the development of new treatment strategies for HCC based on the possible hypothesis.
REFERENCES
1.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55(2):74–108. [CrossRef]
[Pubmed]

2.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71(3):209–49. [CrossRef]
[Pubmed]

3.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17–48. [CrossRef]
[Pubmed]

4.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16(10):589–604. [CrossRef]
[Pubmed]

5.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7(1):6. [CrossRef]
[Pubmed]

6.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365(12):1118–27. [CrossRef]
[Pubmed]

7.
Guan MC, Wang MD, Liu SY, et al. Early diagnosis and therapeutic strategies for hepatocellular carcinoma: From bench to bedside. World J Gastrointest Oncol 2021;13(4):197–215. [CrossRef]
[Pubmed]

8.
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35(3):421–30. [CrossRef]
[Pubmed]

9.
Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42(5):1208–36. [CrossRef]
[Pubmed]

10.
Jeong YY, Mitchell DG, Kamishima T. Small (<20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: Clinical implications. AJR Am J Roentgenol 2002;178(6):1327–34. [CrossRef]
[Pubmed]

11.
Levy I, Greig PD, Gallinger S, Langer B, Sherman M. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg 2001;234(2):206–9. [CrossRef]
[Pubmed]

12.
Hussain S, Mubeen I, Ullah N, et al. Modern diagnostic imaging technique applications and risk factors in the medical field: A review. Biomed Res Int 2022;2022:5164970. [CrossRef]
[Pubmed]

13.
Cole WH, Everson TC. Spontaneous regression of cancer: Preliminary report. Ann Surg 1956;144(3):366–83. [CrossRef]
[Pubmed]

14.
Cole WH. Spontaneous regression of cancer and the importance of finding its cause. Natl Cancer Inst Monogr 1976;44:5–9.
[Pubmed]

15.
Johnson FL, Lerner KG, Siegel M, et al. Association of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet 1972;2(7790):1273–6. [CrossRef]
[Pubmed]

16.
Meza-Junco J, Montaño-Loza AJ, Martinez-Benítez B, Cabrera-Aleksandrova T. Spontaneous partial regression of hepatocellular carcinoma in a cirrhotic patient. Ann Hepatol 2007;6(1):66–9.
[Pubmed]

17.
Chang WY. Complete spontaneous regression of cancer: Four case reports, review of literature, and discussion of possible mechanisms involved. Hawaii Med J 2000;59(10):379–87.
[Pubmed]

18.
Mahajan A, Shetty A, Koteshwar P, Musunuri B, Shetty S, Bhat G. Complete regression of hepatocellular carcinoma with low dose of sorafenib. J Clin Exp Hepatol 2021;11(6):756–57. [CrossRef]
[Pubmed]

19.
Toyoda H, Sugimura S, Fukuda K, Mabuchi T. Hepatocellular carcinoma with spontaneous regression of multiple lung metastases. Pathol Int 1999;49(10):893–7. [CrossRef]
[Pubmed]

20.
Jeon SW, Lee MK, Lee YD, et al. Clear cell hepatocellular carcinoma with spontaneous regression of primary and metastatic lesions. Korean J Intern Med 2005;20(3):268–73. [CrossRef]
[Pubmed]

21.
Nam SW, Han JY, Kim JI, et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol 2005;20(3):488–92. [CrossRef]
[Pubmed]

22.
Terasaki T, Hanazaki K, Shiohara E, Matsunaga Y, Koide N, Amano J. Complete disappearance of recurrent hepatocellular carcinoma with peritoneal dissemination and splenic metastasis: A unique clinical course after surgery. J Gastroenterol Hepatol 2000;15(3):327–30. [CrossRef]
[Pubmed]

23.
Alqutub A, Peck D, Marotta P. Spontaneous regression of a large hepatocellular carcinoma: Case report. Ger Med Sci 2011;9:Doc07. [CrossRef]
[Pubmed]

24.
Blondon H, Fritsch L, Cherqui D. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: Possible role of immune mechanisms. Eur J Gastroenterol Hepatol 2004;16(12):1355–9. [CrossRef]
[Pubmed]

25.
Yano Y, Yamashita F, Kuwaki K, et al. Partial spontaneous regression of hepatocellular carcinoma: A case with high concentrations of serum lens culinaris agglutinin-reactive alpha fetoprotein. Kurume Med J 2005;52(3):97–103. [CrossRef]
[Pubmed]

26.
Ohtani H, Yamazaki O, Matsuyama M, et al. Spontaneous regression of hepatocellular carcinoma: Report of a case. Surg Today 2005;35(12):1081–6. [CrossRef]
[Pubmed]

27.
Imaoka S, Sasaki Y, Masutani S, et al. Necrosis of hepatocellular carcinoma caused by spontaneously arising arterial thrombus. Hepatogastroenterology 1994;41(4):359–62.
[Pubmed]

28.
Anthony PP. Hepatocellular carcinoma: An overview. Histopathology 2001;39(2):109–18. [CrossRef]
[Pubmed]

29.
Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 1998;43(4):575–7. [CrossRef]
[Pubmed]

30.
Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: Molecular biology. Int J Clin Oncol 2010;15(6):544–51. [CrossRef]
[Pubmed]

31.
Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998;27(2):407–14. [CrossRef]
[Pubmed]

32.
Xu X, Tan Y, Qian Y, et al. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2019;98(2):e13923. [CrossRef]
[Pubmed]

33.
Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: An early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61(3):427–38. [CrossRef]
[Pubmed]

34.
Ramzan M, Sturm N, Decaens T, et al. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int 2016;36(3):434–44. [CrossRef]
[Pubmed]

35.
Storey RE, Huerta AL, Khan A, Laber DA. Spontaneous complete regression of hepatocellular carcinoma. Med Oncol 2011;28(4):948–50. [CrossRef]
[Pubmed]

36.
Gottfried EB, Steller R, Paronetto F, Lieber CS. Spontaneous regression of hepatocellular carcinoma. Gastroenterology 1982;82(4):770–4.
[Pubmed]

37.
Zheng GY, Xin HL, Li B, Xu YF, Yi TJ, Ling CQ. Total saponin from root of Actinidia valvata Dunn prevents the metastasis of human hepatocellular carcinoma cells. Chin J Integr Med 2012;18(3):197–202. [CrossRef]
[Pubmed]

38.
Liu XL, Wang H, Zhang L, et al. Anticancer effects of crude extract from Melia toosendan Sieb. et Zucc on hepatocellular carcinoma in vitro and in vivo. Chin J Integr Med 2016;22(5):362–9. [CrossRef]
[Pubmed]

39.
Gao JZ, DU JL, Wang YL, Li J, Wei LX, Guo MZ. Synergistic effects of curcumin and bevacizumab on cell signaling pathways in hepatocellular carcinoma. Oncol Lett 2015;9(1):295–9. [CrossRef]
[Pubmed]

40.
Li X, Lin Z, Zhang B, et al. ?-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci Rep 2016;6:21010. [CrossRef]
[Pubmed]

41.
Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of interleukin 18. Am J Gastroenterol 2002;97(3):774–5. [CrossRef]
[Pubmed]

42.
Jozuka H, Jozuka E, Suzuki M, Takeuchi S, Takatsu Y. Psycho-neuro-immunological treatment of hepatocellular carcinoma with major depression – A single case report. Curr Med Res Opin 2003;19(1):59–63. [CrossRef]
[Pubmed]

43.
Mochizuki T, Takehara Y, Nishimura T, Takahashi M, Kaneko M. Regression of hepatocellular carcinoma. AJR Am J Roentgenol 1991;156(4):868–9. [CrossRef]
[Pubmed]

44.
Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford) 2012;14(8):500–5. [CrossRef]
[Pubmed]

45.
Kumar A, Le DT. Hepatocellular carcinoma regression after cessation of immunosuppressive therapy. J Clin Oncol 2016;34(10):e90–2. [CrossRef]
[Pubmed]

46.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8(9):1069–86. [CrossRef]
[Pubmed]

47.
Ota Y, Aso K, Otake S, et al. Contrast-enhanced ultrasonography for the diagnosis of spontaneous necrosis of hepatocellular carcinoma: A report of 2 cases. Radiol Case Rep 2022;18(1):173–81. [CrossRef]
[Pubmed]

48.
Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev 2003;8(3):303–18.
[Pubmed]

49.
Yaguchi M, Miyazawa K, Otawa M, et al. Vitamin K2 selectively induces apoptosis of blastic cells in myelodysplastic syndrome: Flow cytometric detection of apoptotic cells using APO2.7 monoclonal antibody. Leukemia 1998;12(9):1392–7. [CrossRef]
[Pubmed]

50.
Nishimaki J, Miyazawa K, Yaguchi M, et al. Vitamin K2 induces apoptosis of a novel cell line established from a patient with myelodysplastic syndrome in blastic transformation. Leukemia 1999;13(9):1399–405. [CrossRef]
[Pubmed]

51.
Miyazawa K, Nishimaki J, Ohyashiki K, et al. Vitamin K2 therapy for myelodysplastic syndromes (MDS) and post-MDS acute myeloid leukemia: Information through a questionnaire survey of multi-center pilot studies in Japan. Leukemia 2000;14(6):1156–7. [CrossRef]
[Pubmed]

52.
Nouso K, Uematsu S, Shiraga K, et al. Regression of hepatocellular carcinoma during vitamin K administration. World J Gastroenterol 2005;11(42):6722–4. [CrossRef]
[Pubmed]

53.
Johnson FL, Lerner KG, Siegel M, et al. Association of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet 1972;2(7790):1273–6. [CrossRef]
[Pubmed]

54.
McCaughan GW, Bilous MJ, Gallagher ND. Long-term survival with tumor regression in androgen-induced liver tumors. Cancer 1985;56(11):2622–6. [CrossRef]
[Pubmed]

55.
Franses JW, Bhan I, Pankaj A, Ting DT, Deshpande V, Tanabe K. Spontaneous immune-mediated regression of hepatocellular carcinoma with high tumor mutational burden. JCO Precis Oncol 2021;5:PO.21.00092. [CrossRef]
[Pubmed]

56.
Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 2019;30(1):44–56. [CrossRef]
[Pubmed]

57.
Ang C, Klempner SJ, Ali SM, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019;10(40):4018–25. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
RBK would like to thank SGG for proof reading, AKK and ABK for all the help.
Author ContributionsRajendra Benny K - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Vinitha Tony - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sonia Thanikaivelu - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ravish Sanghi Raju - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rijo Isaac NP - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bedanta Barman - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Allen Kiruba - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rohan Samuel Thomas - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Neenu Oliver John - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Arvind Sathyamurthy - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jeba Karunya Ramireddy - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thomas Samuel Ram - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Rajendra Benny K et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.