|
Research Article
Antiplatelet therapy after pancreaticoduodenectomy with portal vein resection as the optimal preventative strategy for maintaining primary vein patency
1 Division of Hepatopancreatobiliary Surgery, Department of Surgery, Carolinas Medical Center, Atrium Health, Charlotte, NC, USA
2 Resident, Division of Hepatopancreatobiliary Surgery, Carolinas Medical Center, Atrium Health, Charlotte, NC, USA
3 Attending Surgeon, Division of Hepatopancreatobiliary Surgery, Carolinas Medical Center, Atrium Health, Charlotte, NC, USA
Address correspondence to:
Dionisios Vrochides
MD, PhD, FACS, FRCSC, Division of Hepatopancreatobiliary Surgery, Department of Surgery, Carolinas Medical Center, Atrium Health, Charlotte, NC 28203,
USA
Message to Corresponding Author
Article ID: 100096Z04MA2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Anderson MK, Pickens RC, Davis J, Baker EH, Martinie JB, Iannitti DA, Vrochides D. Antiplatelet therapy after pancreaticoduodenectomy with portal vein resection as the optimal preventative strategy for maintaining primary vein patency. Int J Hepatobiliary Pancreat Dis 2021;11:100096Z04MA2021.ABSTRACT
Aim: Anticoagulation after pancreaticoduodenectomy with portal vein resection is relevant to maintaining vein patency; however, no uniformly accepted algorithm exists for anticoagulant selection. We evaluated patients undergoing pancreaticoduodenectomy with various degrees of portal venous resection and reconstruction to determine the optimal regimen for anticoagulation therapy.
Methods: A retrospective review of 51 patients with pancreatic adenocarcinoma who underwent pancreaticoduodenectomy with venous resection was performed (2006 through 2016). Venous resections were categorized as tangential, segmental with primary anastomosis, or segmental with vein graft. Type of anticoagulation selected by the surgeon was noted. The primary outcome was vein patency measured through the first year postoperatively.
Results: Of 33 patients eligible for study, 7 underwent tangential resection, 16 underwent segmental resection, and 10 underwent vein graft. Vein patency rates at 2, 4, 6, and 11 months were 96.9%, 93.1%, 89.3%, and 62.5%, respectively. All patients with tangential resection showed patency at six months, regardless of the use of anticoagulation or not. For segmental resection, patency was higher with antiplatelet/warfarin (62.5%) compared with no treatment (25%). For segmental resection with vein graft, patency at 10 months was higher with antiplatelet therapy (80%) compared with warfarin (33%).
Conclusion: For patients undergoing pancreaticoduodenectomy with portal vein resection, anticoagulation therapy may be guided by the degree of resection and reconstruction required. Although anticoagulation therapy may be unnecessary with tangential vein resection, anticoagulation in the form of antiplatelet therapy may be preferable in patients who have segmental vein resections with primary anastomoses and vein grafts.
Keywords: Anticoagulation, Pancreatic adenocarcinoma, Portal vein resection, Whipple
Introduction
The optimal anticoagulation strategy after pancreaticoduodenectomy (PD) with portal venous resection remains a challenging issue in the postoperative setting. The balance of the risks of postoperative hemorrhage with the loss of portal venous patency are primarily what drives this discussion. Additionally, factors such as the extent of vein resection and the variety of available anticoagulant agents further complicate decision-making.
The incidence of hemorrhagic complications after PD with portal venous resection ranges from 7% to 8%, and is associated with an increased risk of postoperative bleeding, complications, and reoperation as compared to PD without vein resection [1],[2],[3]. Meanwhile, portal venous stenosis or thrombosis after PD predisposes patients to portal hypertension, variceal development, and splanchnic venous infarction [4],[5]. In patients undergoing PD with venous resection for pancreatic adenocarcinoma, major postoperative morbidity may increase the length of stay, delay the initiation of adjuvant systemic therapy, and ultimately result in poorer long-term outcomes [6].
The role of postoperative anticoagulation therapy after PD with venous resection has received limited attention in the published literature. Smoot et al. reported a rate of postoperative portal venous thrombosis of 17%, with no difference in thrombosis rates between patients receiving anticoagulation versus those who did not [7]. Other authors have reported postoperative portal venous thrombosis rates of 5–24% [8],[9]. Unfortunately, these studies did not report the patency rate relative to the type of portal venous resection and anticoagulation regimen.
For patients who undergo PD at our institution, there is currently no protocol to guide postoperative anticoagulation. In our current practice, antiplatelet therapy is typically utilized for tangential vein resections and segmental vein resections with primary anastomosis, whereas therapeutic anticoagulation (enoxaparin or warfarin) is used for segmental resections with an interposition graft. We aimed to evaluate the outcomes, specifically primary vein patency, of patients undergoing PD with venous resection with respect to anticoagulation.
MATERIALS AND METHODS
Patient characteristics
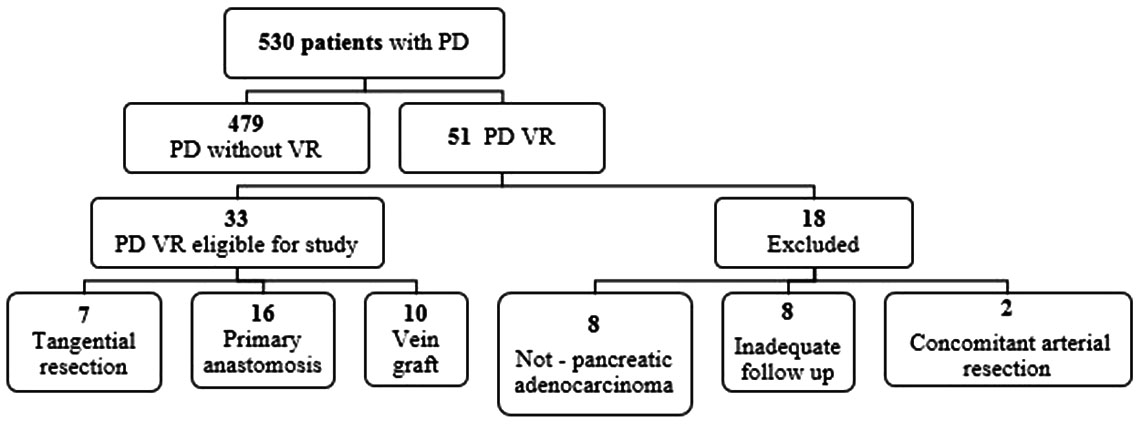
A retrospective review of patients undergoing PD within the division of hepatopancreatobiliary (HPB) surgery at a single institution (Carolinas Medical Center, Charlotte, NC, USA) was performed from 2006 through 2016. The study was approved by the Institutional Review Board for the institution (06-12-34E). All patients who undergo major operations within the division of HPB are tracked using an institutional HIPAA-compliant patient database and data was obtained from this database and specific retrospective review of the electronic medical records. Of the 530 patients identified in the database who underwent PD during the study period, 51 underwent surgery for pancreatic adenocarcinoma and required a concomitant resection and repair/reconstruction of the portal vein (Figure 1). Patients were excluded from the study if they underwent an operation for an indication other than pancreatic adenocarcinoma (e.g., benign disease, pancreatic neuroendocrine tumor, or cholangiocarcinoma, n=8), concomitant arterial resection (n=2), or if follow-up from the electronic medical record was incomplete (n=8). The remaining eligible 33 patients were divided into three groups: tangential vein resection (TR), segmental vein resection with primary anastomosis (SR), and segmental vein resection with vein graft (VG).
Preoperative assessment
Patients were initially evaluated at the Hepatopancreatobiliary (HPB) Surgery Clinic. A thin-slice (4 mm) triple-phase computed tomography (CT) scan of the abdomen was obtained to characterize the lesion, and a CT scan of the chest was obtained to rule out thoracic metastases. The carbohydrate antigen (CA) 19-9 level was measured. An endoscopic ultrasound with fine needle aspiration was performed to confirm presence of pancreatic adenocarcinoma. Patients were staged according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines [10]. Neoadjuvant chemotherapy was offered to those patients who were staged as borderline resectable or locally advanced tumors. No patients received neoadjuvant radiation therapy. If the patient accepted this approach, they underwent a diagnostic laparoscopy to evaluate for grossly disseminated intra-abdominal metastases before proceeding with neoadjuvant therapy. After completion of neoadjuvant therapy, a CT scan was obtained to evaluate progression of disease before proceeding with surgical resection. If the patient declined neoadjuvant chemotherapy, they underwent a diagnostic laparoscopy; a surgical resection was performed in the absence of grossly disseminated intra-abdominal metastases.
Operative procedure
Pancreaticoduodenectomy was performed by a specialty trained HPB surgeon. The pancreaticojejunostomy was performed in duct to mucosa fashion. The tumor–vascular interface was approached as the last step of the procedure and vascular resections were performed as follows:
Tangential vein resection (TR): An angled vascular clamp was placed underneath the area of tumor-vein involvement. The vein was cut sharply above the clamp and a primary venorrhaphy was performed.
Segmental vein resection with primary anastomosis (SR): After administration of systemic anticoagulation, vascular clamps were placed above and below the area of venous involvement. The vein was resected en-bloc with the specimen. For a 3 cm or less distance between the portal and superior mesenteric veins, an end-to-end vein to vein anastomosis was performed.
Segmental vein resection with vein graft (VG): After administration of systemic anticoagulation, vascular clamps were placed above and below the area of venous involvement. The vein was resected en-bloc with the specimen. For a greater than 3 cm distance between the portal and superior mesenteric veins, a cadaveric allograft was used for the interposition anastomosis.
Type of anticoagulation therapy
Three postoperative regimens were used as follows:
None: no postoperative anticoagulation was administered.
Antiplatelet therapy: aspirin 325 milligram (mg) tablet once daily for the first postoperative month, followed by aspirin 81 mg daily; this regimen was the standard regimen followed by the faculty surgeons at our institution.
Warfarin therapy: after initiating enoxaparin therapy at 1 mg/kg as a bridging anticoagulation therapy, warfarin was initiated and maintained to an international normalized ratio (INR) of above 2.0.
The type of anticoagulation therapy had been selected at the discretion of the attending surgeon. Anticoagulation therapy was typically initiated on postoperative day two. All patients received postoperative care on a specialty unit that was dedicated to the management of complex HPB diseases.
Outcomes
For each type of vein resection (TR, SR, or VG), outcomes were compared between the types of postoperative anticoagulation administered. Primary measures included vein patency at 2–12 months postoperatively. Vein patency was defined as radiographic presence (based on CT scan) of contrast flow through the vessel. Secondary measures were the short-term postoperative outcomes: Clavien-Dindo grade of 90-day morbidity, pancreatic leak, and length of stay. Pancreatic leak was defined according to the International Study Group for Pancreatic Fistula, which states: “an external fistula with a drain output of any measurable volume after postoperative day 3 with an amylase level greater than three times the upper limit of the normal serum value” [11],[12]. Postoperative bleeding complications were defined as the need for a postoperative (90 day) transfusion of packed red blood cells. Blood was transfused for a postoperative hemoglobin of less than 7 mg/dL or a 4 mg/dL decrease in the hemoglobin. Additionally, long-term outcome measures were recorded, including return to intended oncologic treatment and overall survival.
Statistical analysis
Demographic and perioperative data were described by frequency. The dependent variable, percentage of vein patency, was graphed against time (months) following surgery. The Kaplan-Meier test was used to compare vein patency rates between groups. In the present study, patients were separated by anticoagulation agent and time to maximum follow-up. All statistical analyses were conducted using Stata 13.0 software (StataCorp LP, College Station, TX).
RESULTS
Thirty-three patients met inclusion criteria and were included in the analysis. Seven patients underwent tangential resection, 16 underwent segmental resection with primary anastomosis, and 10 underwent segmental resection with vein graft. Patient demographics and preoperative characteristics are shown in Table 1. The median age was 64 years, and 36.3% of patients received neoadjuvant therapy. Median estimated blood loss was 850 milliliters (mL). Median length of stay was 10 days. Overall morbidity was 45.5%; there were no deaths within 90 days. Median overall survival was 18 months.
Vein patency outcomes
The median radiographic follow-up for the study group was 18 months. Vein patency rates at 2 months, 4 months, 6 months, and 11 months were 96.9%, 93.1%, 89.3%, and 62.5%, respectively. Of 7 patients undergoing PD with TR, 4 received postoperative antiplatelet therapy, and 3 received no anticoagulation therapy. All 4 patients who received antiplatelet therapy demonstrated vein patency at a minimum follow-up of six months. For the 3 remaining patients who received no antiplatelet therapy, all demonstrated vein patency at a minimum of 18 months.
For patients undergoing PD with SR, 10 received antiplatelet therapy, 1 received warfarin, and 5 patients received no anticoagulation (Figure 2A). At four months follow-up, vein patency was 80% for patients who received no antiplatelet therapy, and 81.8% for those who received antiplatelet therapy and/or warfarin.
At 12 months or longer follow-up, a higher percentage of patients who received antiplatelet therapy (aspirin or warfarin) showed vein patency compared with patients who did not receive antiplatelet therapy (62.5% vs 25%, respectively).
For patients undergoing PD with VG, 5 received antiplatelet therapy and 5 received warfarin (Figure 2B). At one month follow-up, vein patency was 100% for both groups. At 10 months follow-up, a higher percentage of patients who received antiplatelet therapy showed vein patency compared with patients who did not receive antiplatelet therapy (80% vs 33%, respectively).
Postoperative blood transfusion
The percentage of patients in our entire group of 33 patients (including TR, SR, and VG) who received a postoperative transfusion of packed red blood cells was 27.3% (n = 9) (Table 2). Two of these patients underwent an operation for re-exploration of hemorrhage. For TR and SR, a higher percentage of patients on postoperative antiplatelet therapy received a postoperative blood transfusion compared to those not on antiplatelet therapy (77% vs 20%, respectively). For VG, a higher percentage of patients on Warfarin therapy received a postoperative blood transfusion compared to those not on antiplatelet therapy.
Return to intended oncologic therapy
For our entire group of 33 patients, 81.8% received adjuvant chemotherapy and/or radiation therapy (Table 3). All patients undergoing TR proceeded to receive adjuvant therapy. For SR and TR, all patients receiving antiplatelet therapy returned for adjuvant therapy.
1Interposition vein graft ? cadaveric vein allograft.
2Antiplatelet therapy = 325 mg aspirin for first 12 weeks, transitioning to 81 mg aspirin. Exception: patients already on chronic anticoagulation resume home regimen without addition of antiplatelet therapy.
Discussion
Identifying the most appropriate postoperative anticoagulation after PD with portal vein resection remains a challenging problem. We aimed to address the lack of evidence for antiplatelet therapy as it relates to the different types of vein resections and reconstructions for patients undergoing PD. In an effort to obtain a homogenous group with an inherently prothrombotic state, we selected patients with pancreatic adenocarcinoma [13]. Our analysis offers the long-term perspective of three different types of portal venous resections and reconstructions and includes vein patency at bi-monthly intervals of two months to one year.
We found that for TR, vein patency rates were high with or without anticoagulation. For patients undergoing PD with SR or VG, antiplatelet therapy appeared to have the optimal vein patency after six months. At 12 months follow-up, vein patency rates for SR with primary anastomosis were 38% higher with antiplatelet therapy compared with no therapy, whereas at 10 months follow-up, vein patency rates for SR with vein graft were 47% higher with antiplatelet therapy compared with warfarin. Although these findings were not statistically significant, perhaps due to the small sample size, they have clinical relevance to patients. To our knowledge, this was the first study to evaluate what form of postoperative anticoagulation was associated with short-term and long-term vein patency after PD with portal venous resection.
This study’s finding of higher rates of vein patency in patients undergoing SR with VG who received antiplatelet therapy compared with traditionally used warfarin/enoxaparin therapy was important. Antiplatelet agents such as aspirin or clopidogrel have generally been applied in the prevention of arterial thrombosis [14],[15]. However, aspirin may have some beneficial effects in the prevention of venous thromboembolism because of a lessening of platelet adhesion and procoagulant conditions [16]. In patients treated with aspirin for venous thromboembolism, Becattini et al. reported a recurrence rate of 6.6% and a only 1% incidence of hemorrhagic complications [17]. Data specifically regarding patency with anticoagulation versus antiplatelet therapy after vein resection and reconstruction for pancreatic malignancy is, not surprisingly, limited. Importantly the inherent differences in administration and required monitoring for the different types of anticoagulation therapy should also be considered as it relates to patient adherence. While warfarin requires frequent laboratory monitoring with titration and enoxaparin requires painful daily injections, antiplatelet therapy with aspirin is a readily accessible generic tablet without the need for monitoring. Without data to suggest a clear superiority of full anticoagulation (e.g., warfarin or enoxaparin) as suggested by this study, these alternate benefits of antiplatelet therapy may support its preferential use.
Based on institutional experience and the data presented in this study, the authors propose a new pathway for postoperative anticoagulation after pancreaticoduodenectomy with venous resection (Figure 2). The authors do not recommend any anticoagulation therapy for tangential vein resections. For segmental vein resections with primary anastomoses, the authors recommend 12 weeks of initial antiplatelet therapy (initiated on postoperative day 2) with 325 mg aspirin daily, transitioning to 81 mg daily. For segmental vein resections with cadaveric vein allografts, the authors also recommend the same antiplatelet regimen. Specific note is made for patients with a known preoperative hypercoagulable condition (aside from malignancy) with pre-existing anticoagulation therapy, who should continue anticoagulation therapy as previously prescribed without additional antiplatelet therapy.
Although this study addresses a relevant topic in pancreatic surgery, there were limitations. Given the small sample size of our study, a type II error was likely. Eight of the 18 patients excluded from the study lacked follow-up, but none of these patients expired within 90 days of the operation or as a result of the operation. It is also plausible that portal vein patency after PD with portal vein resection may be a function of inherent patient-related factors and tumor biology rather than anticoagulation itself. Given the high incidence of local recurrence in patients who undergo an operation for pancreatic adenocarcinoma, residual tumor burden could promote a persistent hypercoagulable state even after the procedure [18]. Given our preliminary findings, a prospective cohort study of patients undergoing PD with venous resection that evaluates outcomes related to postoperative antiplatelet therapy with different regimens, including novel, more expensive agents such as rivaroxaban and dabigatran, may be useful.
Conclusion
For patients undergoing PD with portal vein resection, the choice of anticoagulation therapy may be guided by the degree of resection and reconstruction required. Although anticoagulation therapy may be unnecessary after PD with tangential vein resection, anticoagulation in the form of antiplatelet therapy may be preferable in patients who have segmental vein resections with primary anastomoses and vein grafts. Given the clinical relevance of this topic, a larger prospective trial would prove beneficial in delineating the benefit of anticoagulation after portal vein resection in patients undergoing PD.
REFERENCES
1.
Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg 2006;10(8):1106–15. [CrossRef]
[Pubmed]

2.
Zhou Y, Zhang Z, Liu Y, Li B, Xu D. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: A meta-analysis. World J Surg 2012;36(4):884–91. [CrossRef]
[Pubmed]

3.
Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103(3):179–91. [CrossRef]
[Pubmed]

4.
Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology 2010;51(1):210–8. [CrossRef]
[Pubmed]

5.
Parikh S, Shah R, Kapoor P. Portal vein thrombosis. Am J Med 2010;123(2):111–9. [CrossRef]
[Pubmed]

6.
Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21(9):2873–81. [CrossRef]
[Pubmed]

7.
Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg 2006;10(10):1371–5. [CrossRef]
[Pubmed]

8.
Chu CK, Farnell MB, Nguyen JH, et al. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: A multicenter analysis. J Am Coll Surg 2010;211(3):316–24. [CrossRef]
[Pubmed]

9.
Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: Margin status and survival duration. J Gastrointest Surg 2004;8(8):935–50. [CrossRef]
[Pubmed]

10.
Tempero MA, Malafa MP, Al-Hawary M. Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15(8):1028–61. [CrossRef]
[Pubmed]

11.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250(2):187–96. [CrossRef]
[Pubmed]

12.
Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005;138(1):8–13. [CrossRef]
[Pubmed]

13.
Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293(6):715–22. [CrossRef]
[Pubmed]

14.
Cadroy Y, Bossavy JP, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation 2000;101(24):2823–8. [CrossRef]
[Pubmed]

15.
Halkes PHA, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A; ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet 2006;367(9523):1665–73. [CrossRef]
[Pubmed]

16.
Poujol-Robert A, Boëlle PY, Conti F, et al. Aspirin may reduce liver fibrosis progression: Evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol 2014;38(5):570–6. [CrossRef]
[Pubmed]

17.
Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366(21):1959–67. [CrossRef]
[Pubmed]

18.
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001;358(9293):1576–85. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Meshka K Anderson - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ryan C Pickens - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joshua Davis - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Erin H Baker - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
John B Martinie - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
David A Iannitti - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dionisios Vrochides - Conception of the work, Design of the work, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Meshka K Anderson et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.