|
|
Original Article
| ||||||
| Advantage of endoscopic-ultrasound-fine-needle aspiration associated to Sendai clinical guidelines in detecting the malignant risk in patients with undetermined pancreatic cysts: Long-term follow-up | ||||||
| Pietro Gambitta1, Paolo Aseni2, Paola Fontana1, Emilia Bareggi1, Edoardo Forti4, Alberto Tringali4, Francesco Molteni3, Maurizio Vertemati5 | ||||||
|
1Unità Operativa di Gastroenterologia ed Endoscopia Digestiva Ospedale Luigi Sacco, Milano, Italy.
2Dipartimento di Emergenza Urgenza Medicina d'Urgenza e Pronto Soccorso, ASST, Grande Ospedale Metropolitano Niguarda, Milano, Italy. 3Università Statale di Milano, Dipartimento di Scienze Sociali e Politiche, Milano, Italy. 4Endoscopia Digestiva e Interventistica, Ospedale Niguarda Ca' Granda, Milano, Italy. 5Dipartimento di Scienze Biomediche e Cliniche "L. Sacco" Università degli Studi di Milano, Italy. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Gambitta P, Aseni P, Fontana P, Bareggi E, Forti E, Tringali A, Molteni F, Vertemati M. Advantage of endoscopic-ultrasound-fine-needle aspiration associated to Sendai clinical guidelines in detecting the malignant risk in patients with undetermined pancreatic cysts: Long-term follow-up. Int J Hepatobiliary Pancreat Dis 2016;6:100–107. |
|
Abstract
|
|
Aims:
Contradictory information exists on whether different clinical guidelines are effective in detecting the malignant risk in patients with pancreatic cysts. We have retrospectively evaluated the accuracy and the long-term outcome in patients with pancreatic cysts with a diameter ≥ 2 cm when indication for surgery was established by clinical evaluation of their malignant risk according to Sendai Clinical Guidelines associated to endoscopic-ultrasound-fine-needle aspiration.
Material and Methods: Patients with pancreatic cysts with a diameter ≥2 cm were evaluated for their potential malignant risk by endoscopic-ultrasound-fine-needle aspiration associated to the clinical evaluation by Sendai Clinical Guidelines. Long-term outcome and comparison in patients survival as well as the accuracy in detecting malignancies were evaluated with the combined clinical and endoscopic evaluation. Results: Two hundred eighteen patients with pancreatic cysts were observed during a nine-year period of the study and 74 of them (33.9%) presenting with a pancreatic cyst ≥2 cm were eligible for the study. Fourteen malignant neoplasms (18.9%) were detected. The accuracy in detecting malignancy of combined clinical and endoscopic evaluation was very high (0.99). The five-year survival rates for patients who underwent surgery with benign and malignant pancreatic cysts and for patients in observational follow-up were similar (70% and 85%). The cohort of patients with malignant pancreatic cysts with ductal adenocarcinoma showed a five-year survival rate of 41%. Conclusion: Endoscopic ultrasound fine-needle aspiration associated to Sendai clinical guidelines showed a high accuracy in detecting malignant risk in patients with pancreatic cysts with a diameter ≥ 2 cm. allowing appropriate selection for surgical treatment with satisfactory long-term survival. | |
|
Keywords:
Diagnostic imaging, Pancreatic cancer, Pancreatic carcinoma, Pancreatic neoplasm, Pancreatic pseudocyst
| |
|
Introduction
| ||||||
|
With the increasing current use of advanced abdominal images modalities such as cross-sectional imaging modalities by computed tomography (CT) scan and magnetic resonance imaging (MRI) scan, pancreatic cysts are commonly encountered. As these lesions have become a common finding, the different types of pancreatic cysts pose a challenging diagnostic dilemma to assess the potential for malignancy within a cyst [1] [2]. Whereas some lesions show benign behavior, such as serous cystadenomas (SCA) and pancreatic pseudocysts (PPC), others have an unequivocal malignant potential, such as mucinous cystic neoplasm (MCN), main duct (MD) or mixed type (MT) intraductal papillary mucinous neoplasm (MD/MT-IPMN), solid pseudo-papillary neoplasm (SPPN), pancreatic neuroendocrine neoplasm (PNEN) and, to a lesser extent, some branch duct IPMN (BD-IPMN). Endoscopic-ultrasound-fine-needle aspiration (EUS-FNA), allows for analysis of the cyst content, has been increasingly shown to improve the preoperative diagnosis in the majority of patients with undetermined pancreatic cysts. The overall accuracy rates of EUS in differentiating neoplastic versus non-neoplastic lesions range from 40–93%, so EUS imaging features alone for pancreatic cysts seem insufficient to make a diagnosis [3]. The 2006 Sendai Consensus Guidelines (SCG) [4] and the revised Fukuoka Consensus Guidelines (FCG) in 2012 established that patients with presumed but not proven mucinous cystic neoplasm should undergo surgical resection when high-risk stigmata are present [5]. However, these recommendations were established for mucinous cystic neoplasm and not for all pancreatic cysts [6]. Other guidelines were suggested by the American College of Gastroenterologist (ACG) in 2007 [7] and more recently, in 2013, the European Expert Consensus (EEC) [8] stated that unless major contraindications were present, surgical resection should be considered in all symptomatic patients and in patients with MCN and MD/MT-IPMN with high-risk stigmata or with evidence of some defined "worrisome features". Recently, the American Gastroenterological Association (AGA) revised their previous guidelines, which were labeled as evidence-based rather than consensus-based [9]. However, some investigators have expressed concern over whether adopting the AGA guidelines will result in low accuracy in identifying advanced neoplasia [10] [11]. Although outcomes after pancreatic surgery have improved over the last decades, this type of surgery still remains complex, with high morbidity and mortality ranging from 2–15% [12]. In contrast to ductal adenocarcinoma, cystic neoplasms with malignant potential are slow-growing, and a more favorable prognosis has been reported for these neoplasms, even in the setting of malignant degeneration [13]. Efforts to effectively and correctly identify those patients who might benefit from surgery and to identify other patients who would benefit from surveillance without therapy lack evidence by survival comparisons for the different classes of risk. We hypothesize that the EUS-FNA will yield high positive and high negative predictive values when applied to unselected consecutive patients affected by pancreatic cysts with a minimum diameter ≥ 2 cm. The primary aim of this study was to critically evaluate the clinical utility and accuracy of the EUS-FNA in association with the SCG in malignant risk prediction of all pancreatic cysts. The secondary aim of this study was to evaluate the natural course of all patients with pancreatic cysts describing the outcome of different cohorts of patients and their long-term survival when they were stratified for the presence of benign or malignant pancreatic cysts, and when they were submitted to surgical treatment or to clinical surveillance without treatment. | ||||||
|
Materials and Methods
| ||||||
|
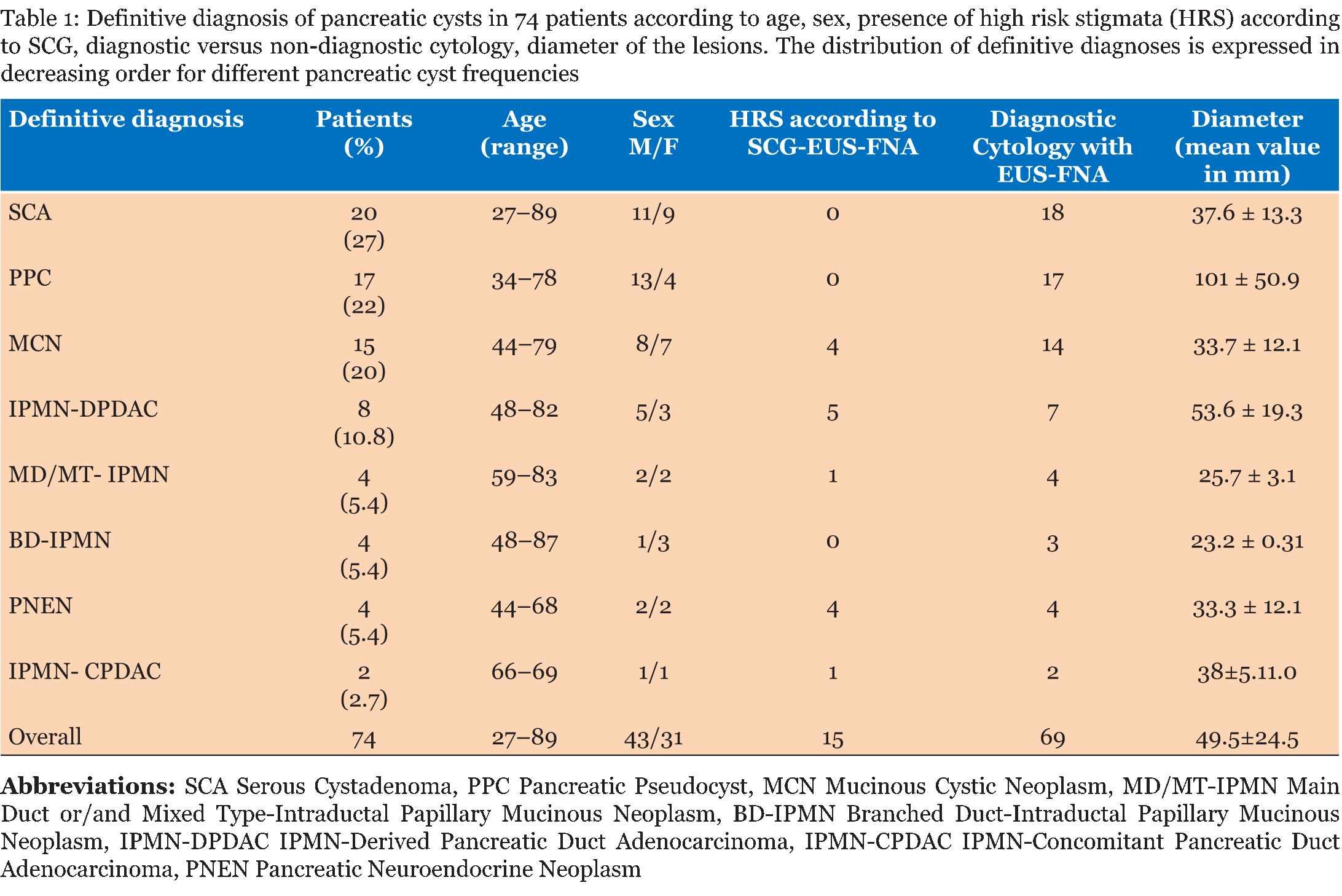
Patients Patients with pancreatic cysts smaller < 2 cm were excluded from the study and underwent a six-month based follow-up. A prospective database with all clinical and radiological data was first created in 2007 according the SCG. Furthermore all morphological, biochemical and cytological findings available with EUS-FNA were also recorded in the same database. At the end of the study all clinical and pathological features of the 74 patients were retrospectively reviewed by a multidisciplinary team. Stratification of malignant risk according to Sendai consensus guidelines Stratification of malignant risk and the EUS-FNA procedure According to Pitman's criteria [14] cytology was graded as: stage I-II-III-IV: non-diagnostic, atypical, negative for malignant and neoplastic benign; stage V, suspicious for malignancy; stage VI, positive for malignancy. Based on these findings, only patients with cytology at stage V and VI were considered at high risk and were considered possible candidates for curative surgery. When none of these criteria were present (stage I, II, III, IV) patients were considered at low risk and submitted to a 3–6 months interval of surveillance depending on the cyst size and on the clinical course. Patients with SCG considered at high risk but with adequate cytology negative for malignant cells were considered at low risk and received 3-months based follow-up. The technique of EUS-FNA has been previously described in detail [15]. The fine needle biopsy procedure was repeated until sufficient material was aspirated. The needles normally used were the same as those for solid lesions, 19 and 22 gauge. Definitive diagnosis Classification of pancreatic cysts Indication for surgery Despite some patients were considered at low risk surgery was considered in those patients with major symptoms suffering from recurrent abdominal pain or back pain unrelated to other causes, or in the presence of recurrent pancreatitis, worsening diabetes, jaundice and weight loss or gastro-duodenal outlet obstruction due to extrinsic compression by the pancreatic cyst. All other patients with pancreatic cysts who did not meet these criteria or classified at LR underwent clinical surveillance. Outcome evaluation and statistical analyses | ||||||
|
Results | ||||||
|
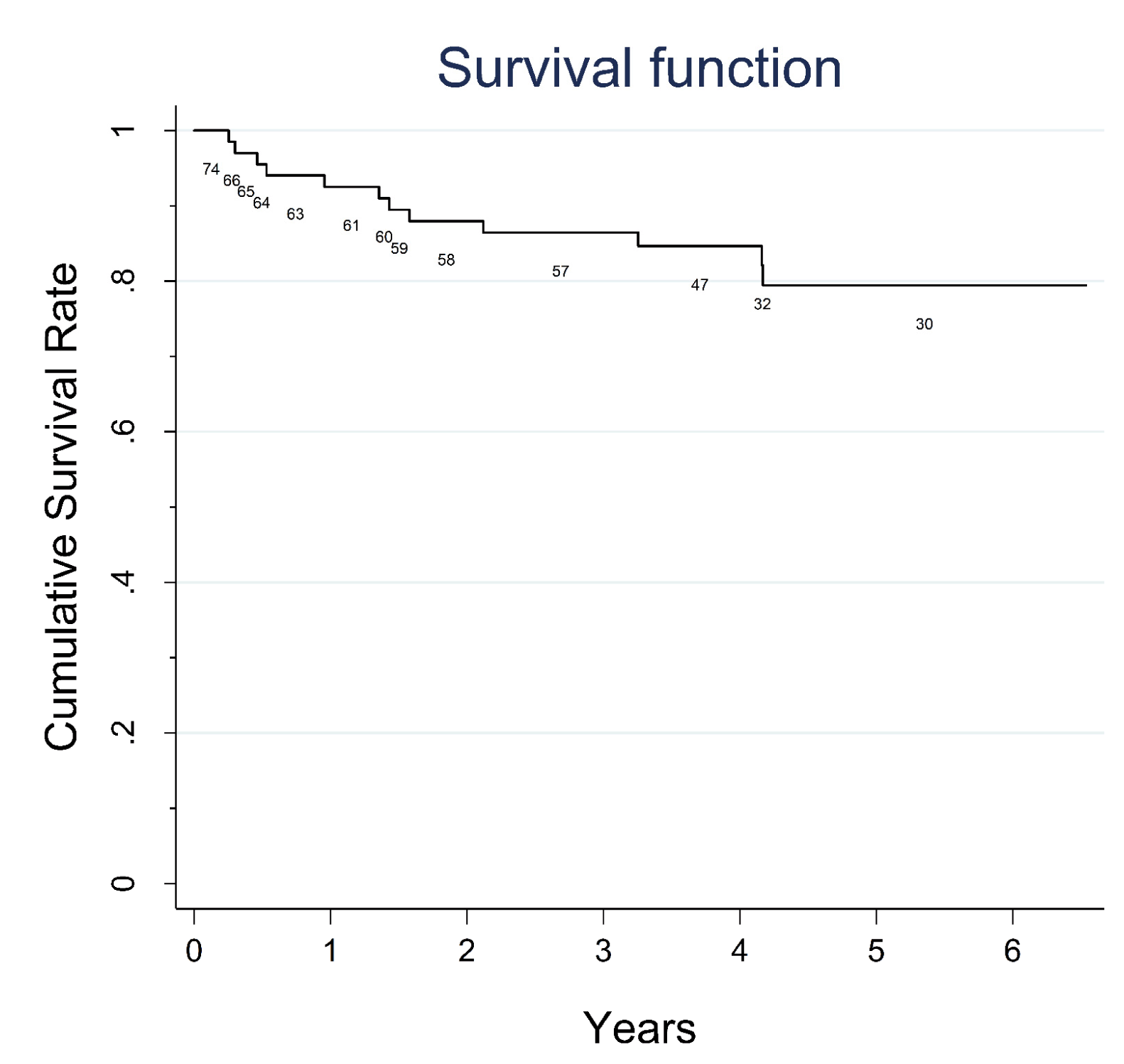
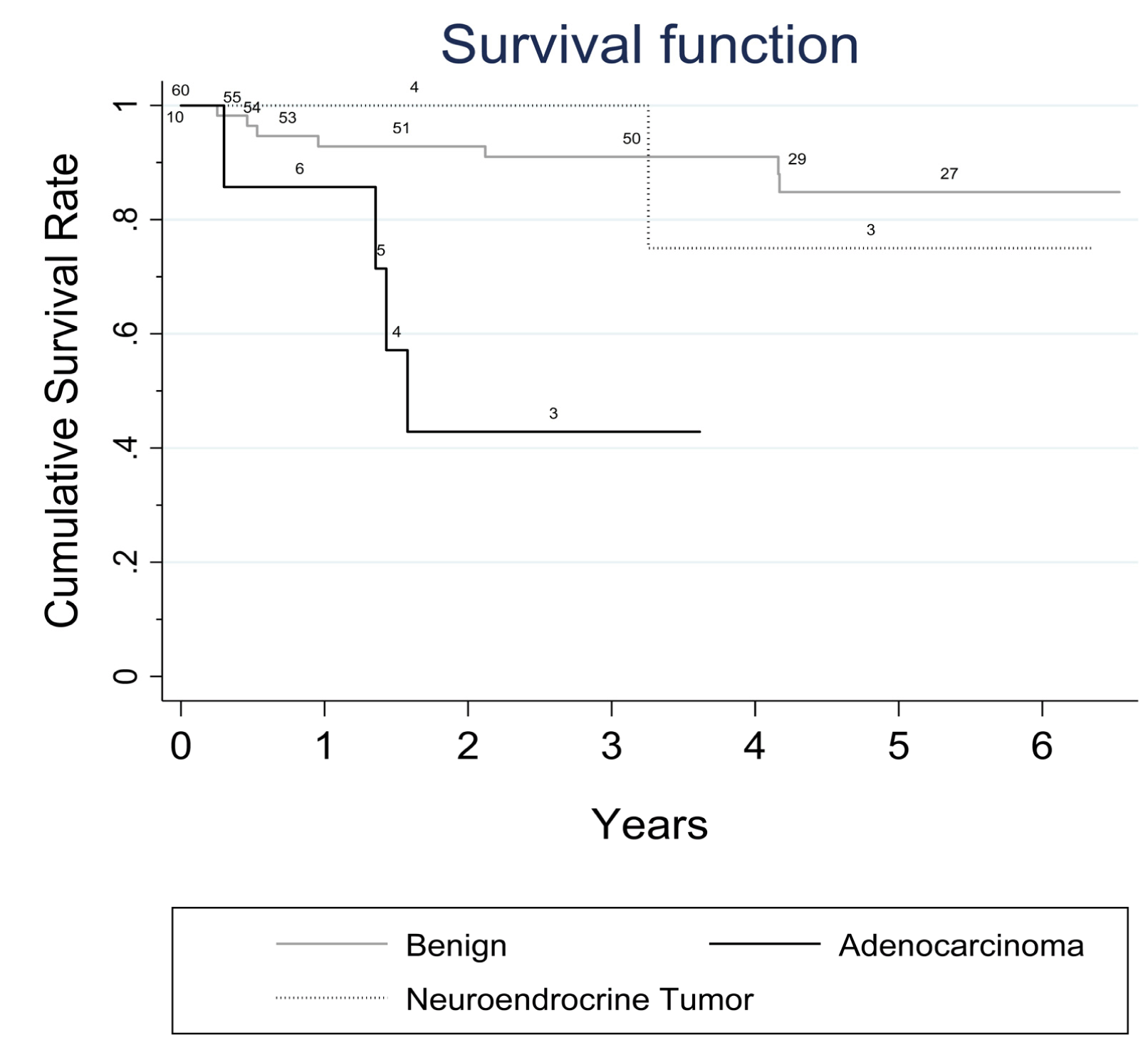
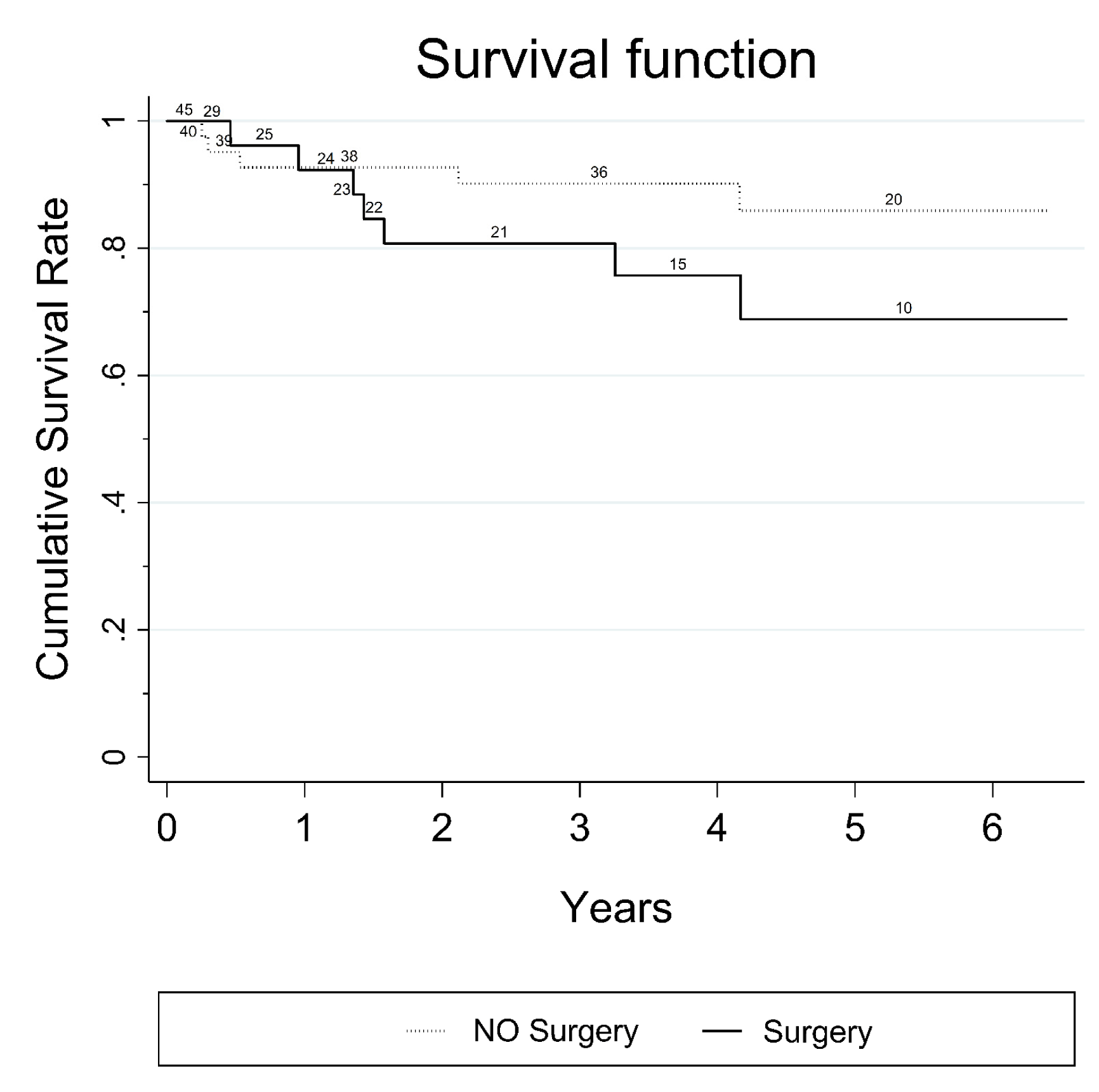
Seventy-four patients presented with 38 pancreatic cysts in the head, 24 in the body and 12 in the tail of the pancreas. Nine patients of 74, originally excluded from the study with a small PC (< 2 cm) showed progression of the PC diameter during the follow-up and were subsequently included in the study. No major complications were registered after the EUS-FNA procedure. According to common terminology criteria for adverse events (CTCAE) four patients had mild early complications: Two patients presented fever and two mild pancreatitis (amylase increased to at least three times the normal values in addition to abdominal pain); one patient had moderate grade 1 complication (intracystic bleeding after complete fluid evacuation). All complications resolved with medical therapy, within three days. Twenty-five patients underwent surgery and histology was obtained from surgical specimen or by surgical biopsy of the lesion. EUS-FNA cytology was diagnostic in 69 of 74 patients. Sufficient fluid for intracystic CEA determination was available only in 31 patients (40%) and was not considered in our analysis. Two patients of 74 with radiological progression of disease who had nondiagnostic cytology, were submitted to a second attempt by EUS-FNA: both patients underwent surgery and only in one patient a malignant cytology could be evidenced. Table 1 summarizes the distribution of different types of PCs for the 74 patients (MCN, SCA, MD/MT- IPMN, BD-IPMN, PPC, IPMN-DPDAC, IPMN-CPDAC and PNEN) according to age, sex and presence of high-risk stigmata evaluated by SCG/EUS-FNA, number of diagnostic cytological diagnoses available, and diameter of the lesions. Accuracy, positive and negative predictive value Malignant pancreatic cysts Surgical treatment Survival | ||||||
|
| ||||||
|
| ||||||
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
Pancreatic cysts represent a wide collection of tumors with different malignant potential at clinical presentation, and the correct choice between surgical excision and follow-up without therapy is a challenging topic of debate. The majority of patients discovered to have a pancreatic cyst is completely asymptomatic and the estimated prevalence in the general population is around 3.5% [19]. Several diagnostic modalities involving cross-sectional radiological imaging or endoscopy are useful for narrowing down the diagnosis and can give evidence to propose surgery or surveillance [20] [21]. However, a definitive diagnosis is often difficult without supporting cytological or histological evidence by means of EUS-FNA or surgical resection. The majority of the guidelines and recommendations proposed during last ten years [4] [5] [6] [7] were designed specifically for the management of MCNs and IPMNs, and the major assumption was that all patients with MD/MT-IPMNs and MCNs with so called "high risk stigmata" according to SCG in 2006 (or "worrisome features" according to FCG in 2012) should be considered for resection, whereas patients with selected non-malignant BD-IPMNs could be observed. However, a preoperative diagnosis of a MCN or IPMN is frequently unavailable, especially via cross-sectional imaging features alone. For that reason, the clinical application of these guidelines remains limited, especially in the initial triage of those patients who present with an incidental pancreatic cyst, due to the difficulty in distinguishing not only between MCNs and IPMNs but also between mucinous pancreatic cysts and other pancreatic cysts, such as SCA, PPC, and PNEN [22] [23]. The first aim of our study was to investigate the potential malignant risk of all undetermined pancreatic cysts with a diameter ≥ 2 cm during a nine-year period with EUS-FNA in association with the conventional clinical and radiological workup proposed by SCG in 2006. The choice to limit our study to patients with pancreatic cysts with a cut-off diameter of ≥ 2 cm, although arbitrary, was suggested by the very low yield of FNA for cysts < 1.5 cm and by the reported lower risk of malignancy for small cysts [24]. When international clinical guidelines are utilized (SCG, FCG, ACG, AGA, EEC) different results have been also reported with lower positive predictive values ranging from 29–66%, [25]. These findings may suggest that if international guidelines were applied during the early triage of patients with PCs, in one out of three patients potentially submitted to surgical resection, no malignancy can be found despite the fact that the hazard ratio of pancreatic cancer risk in those patients has been evaluated to be significantly higher when compared with the rest of patients without cysts [26]. In our study, we registered only one false positive malignant risk evaluation in one symptomatic patient who was submitted to surgery for jaundice and gastric outlet obstruction. The second aim of our study was to follow-up and verify the outcome of all patients with benign and malignant disease as well as the outcome of patients who underwent surgical treatment during the nine-year study period. A considerable overall survival rate for the 14 patients with malignant pancreatic cysts was observed (41% at fifth year for 10 patients with ductal adenocarcinoma and 70% at fifth year for four patients with neuroendocrine tumors). The survival rate of patients with ductal adenocarcinoma was 41%. This was statistically inferior to that observed in patients with benign pancreatic cysts (85% at fifth year). However, the observation of a 41% survival rate for patients with ductal adenocarcinoma might support the fact that the natural course of the malignant pancreatic cysts seems more favorable when compared to that of other form of non cystic presentation of pancreatic adenocarcinoma, in which usually less than 30–40% of patients usually survive after five years [27]. Major technical controversies exist concerning the diagnostic yield of EUS. These lie in the fact that it is virtually impossible to acquire adequate fluid for the examination of cysts less of than 15 mm, and it is impossible to aspirate fluid from, on average, half of the cysts, either because the solid component predominates (as in the case of microcystic SCA) or because the IPMN has a highly viscous content [28]. The use of a 19-and 22-gauge needle was associated with a low rate of complications (6.75%) in accord with other clinical reports [29] and enabled us to obtain adequate diagnostic cytological material in 69 patients of 74 (93%); in the remaining patients the histological surgical specimen was available. A PNEN was found in four patients (5.4%); this finding is similar to that reported in literature (8%) and seems to be clinically relevant, considering that PNENs are relatively rare lesions and that most of these tumors are clinically non-functioning [30]. Pancreatic neuroendocrine neoplasm is a hypoechoic tumor with overt vascularity. Sometimes, this hypoechoic image can be confused with an anechoic cyst. However, the vascularization of the septa in a cyst might be confounding. In the case of cystic degeneration of a PNEN, the differential diagnosis should always require the support of EUS-FNA. | ||||||
|
Conclusion
| ||||||
|
Despite some limitations due to the retrospective nature and to the small sample of patients, our study seems to support the clinical utility of the endoscopic-ultrasound-fine-needle aspiration (EUS-FNA) as a valid diagnostic tool in association to Sendai Consensus Guidelines (SCG) evaluation. Combining clinical evaluation by SCG and endoscopic, morphological and cytological information by EUS-FNA a high accuracy, high positive and high negative predictive value are obtained which allows appropriate selection of patients with suspected malignant pancreatic cysts who can benefit for surgery avoiding unnecessary, high-risk surgical procedures for many other patients. EUS-FNA associated to SCG can be recommended during the early diagnostic stage of patients with pancreatic cysts ≥ 2 cm allowing appropriate selection of those patients with a high malignant risk for surgical treatment with satisfactory long-term survival. Further prospective multicenter studies could better evaluate the real advantage of EUS-FNA with other different proposed clinical guidelines. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Pietro Gambitta – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Paolo Aseni – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Paola Fontana – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Emilia Bareggi – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Edoardo Forti – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Alberto Tringali – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Francesco Molteni – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Maurizio Vertemati – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Pietro Gambitta et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|