| Table of Contents |  |
|
Original Article
| ||||||
| Surgical treatment of severe acute pancreatitis: After 15 years of practice | ||||||
| Jorge Pereira1, Júlio Constantino1, Liliana Duarte2, Helena Pinho3, Luis Pinheiro4 | ||||||
|
1Surgical Specialist, Centro Hospitalar Tondela-Viseu, Viseu, Portugal.
2Surgical Resident, Centro Hospitalar Tondela-Viseu, Viseu, Portugal. 3Surgical Consultant, Centro Hospitalar Tondela-Viseu, Viseu, Portugal. 4Surgical Chief, Centro Hospitalar Tondela-Viseu, Viseu, Portugal. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Pereira J, Constantino J, Duarte L, Pinho H, Pinheiro L. Surgical treatment of severe acute pancreatitis: After 15 years of practice. Int J Hepatobiliary Pancreat Dis 2015;5:74–81. |
|
Abstract
|
|
Aims:
We evaluated the surgical treatment of severe acute pancreatitis by analyzing the surgical methods and clinical outcomes during a 15-year period in our Department of Surgery with the goal of improving our performance. The aim of the study was to investigate the correlation between clinical factors and mortality in these patients.
Methods: The study included the medical records of patients diagnosed with severe acute pancreatitis (Atlanta 2012 classification) who were surgically treated between 2000 and 2014 (15 years) in the Department of Cirurgia 1, Centro Hospitalar Tondela-Viseu in Portugal. The data were statistically analyzed using SPSS® Version 22 IBM® software. Results: A total of 39 patients were included, mostly men with an average age of 58.7 years. The most prevalent etiology was gallstones (41% of cases). The following variables showed an independent relationship with mortality: age, length of stay, Ranson score, APACHE II (acute physiology and chronic health evaluation II) scores at admission and surgery, hematocrit, and renal, respiratory and cardiovascular failures. Conclusion: Findings are similar to those found in previous reports. The Ranson score and APACHE II scores are good prognostic factors for severe acute pancreatitis. Moreover, in this series, APACHE II at surgery appears to be the best predictor of mortality; a cut-off of 21 allowed for an 86.7% sensitivity and a 91% specificity. | |
|
Keywords:
Acute pancreatitis, Multiple organ failure, Necrotizing pancreatitis, Surgery
| |
|
Introduction
| ||||||
|
Acute pancreatitis is a common and usually benign disease with a low overall mortality rate [1]. However, local complications or organ failure can develop in certain patients, resulting in a life-threatening condition with a significantly higher mortality rate [2]. These complicated cases require significantly greater resources, including intensive care and surgery. Severe acute pancreatitis, as defined by consensus at the Atlanta conference in 2012 [3] [4], is a microcirculatory condition leading to pancreatic necrosis; the necrosis is thought to be triggered during the early stages of the inflammatory cascade and is characterized by auto digestion of the gland [5]. The condition can cause abdominal infection, which accounts for 80% [6] of deaths. Surgery is the currently accepted treatment for infection secondary to pancreatic necrosis [7][8], though surgery exhibited little clinical impact on the mortality rate when it was first introduced decades ago [9][10] [11] . Surgery for acute pancreatitis has undergone significant improvement over recent decades. During the 1960s and 1970s, pancreatic resection was advocated as a method of eliminating the inflammation primum movens [7] [10][12]. However, high postoperative mortality resulted in the technique being abandoned. New diagnostic and therapeutic options for pancreatitis emerged beginning in the late 1980s. The advent of computed tomography scan [13] [14] improved the characterization of necrosis and sped up the diagnosis of complications. New broad-spectrum antibiotics with improved penetration into pancreatic tissue were also discovered [5] [12] [15] [16] [17], and the quality of critical care has undergone major improvement. The Atlanta consensus conference in 1992 [18] was important in furthering the overall understanding of pancreatitis and in establishing a common terminology. The consensus standardized the approach and treatment of pancreatitis in treatment centers worldwide and represents a significant step forward. Necrosectomy was introduced as a surgical treatment for acute pancreatitis in the late 1980s [7] [19] [20] and has remained the preferred technique through to the present day. Initially, the technique required a formal laparotomy, but currently, many clinicians advocate minimally invasive or combined techniques [21] [22] [23]. Regardless, necrosectomy remains the gold standard for the treatment of infected pancreatic necrosis. In the early days of its use, necrosectomy was also recommended for sterile necrosis, but this is no longer recommended [7] [24]. Unfortunately, much remains unknown about infected pancreatic necrosis including its origin, factors promoting progression, and the ideal treatment. These questions may take some time to answer, but at present, clinicians must continue to treat affected patients using the best knowledge, technology, and expertise available. In this study, we evaluated the surgical treatment for severe acute pancreatitis by analyzing the surgical methods and clinical outcomes over a 15-year period at our institution with the goal of improving our performance; the aim of the study was to investigate the correlation between clinical factors and mortality in patients submitted to surgical treatment for severe acute pancreatitis | ||||||
|
Materials and Methods
| ||||||
|
Study population Statistical analysis Data were statistically analyzed using SPSS® version 22 IBM® software. The normality of the distribution for each variable was determined using the Shapiro-Wilk test. Variables with a normal distribution were subjected to univariate analysis with the parametric Student t-test, and variables without normal distribution were analyzed with the nonparametric Mann-Whitney test and the chi-square test. For multivariate analysis, a multiple linear model was employed. We then calculated receiver operating characteristic curves for variables that were identified as potential prognostic factors to identify possible cut-off values. Descriptive study Case presentation and progression Most patients (35/39) underwent an abdominal contrast-enhanced computed tomography (CT) scan after a median 3.4 days. Although the median time was generally suitable for performing the first CT scan [4] [13], in some cases, the initial CT scan was performed as long as 18 days after admission. Necrosis was identified in patients undergoing CT scan, defined as a pancreatic parenchymal area without contrast enhancement [4]. Additionally, the patients developing multiple organ failure were identified [4] [31] [32] and classified according to the different organ failures suffered: respiratory, renal, cardiovascular, hematologic, neurologic, hepatic or multiple. Twenty-four of thirty-nine (61.5%) patients were diagnosed with pancreatic infection based on tissue or fluid culture. Gram-negative bacteria were identified in 23.1% of samples; Gram-positive bacteria in 33.3%, and anaerobic species in 5.1%; 38.1% of samples were negative on culture. Preoperative treatment Antibiotic therapy was initiated before or after surgery depending on when infection was diagnosed. Once an infectious agent was identified, the distribution of selected antibiotics differed as follows: carbapenem (66.7%), piperacillin-tazobactam (10.3%), cephalosporin (5.1%), metronidazole combined with a second agent (7.9%), fluoroquinolones (2.6%), and other (2.6%). In addition, 38.5% of patients also received an antifungal agent. Surgical intervention Surgery was performed at different points of evolution of the disease, depending on individual clinical factors. Multiple organ failure and abdominal compartment syndrome were the most common indications in patients who underwent surgery during the first two weeks of disease. Those who were surgically treated after the first two weeks of disease were typically treated because of infection. A total of 24 patients underwent surgery to treat confirmed infection; of these, 41.6% of procedures were performed during the first two weeks of disease. Most patients underwent a median laparotomy, and the remainder underwent transverse subcostal laparotomy. The most common procedure was fluid collection drainage followed by necrosectomy. Abdominal decompression without any additional procedures was performed once in a case of abdominal compartment syndrome. | ||||||
| ||||||
|
| ||||||
| ||||||
|
Results | ||||||
|
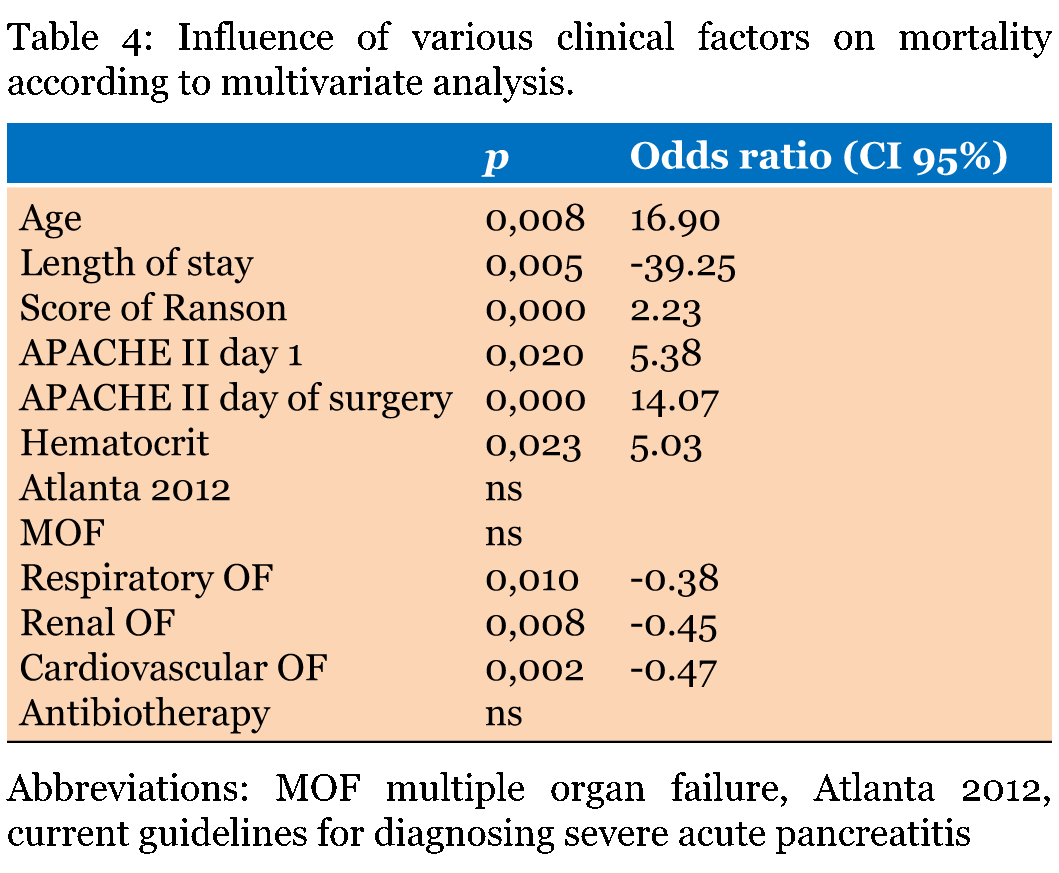
Morbidity and prognostic factors Surgical complications occurred in 38.5% of cases, including intestinal necrosis (17.9%), digestive fistula (10.3%), and hemorrhage (2.6%). Nine of 24 survivors (23.1%) developed severe sequelae, including respiratory dysfunction, incisional hernia, digestive fistula, and dependence in everyday activities. The mean length of stay was 57 days with a maximum of 154 days. The mean length of stay in the intensive care unit (ICU) was 23.5 days with a maximum of 136 days. The length of stay in the ICU did not influence the mortality. The hospital mortality was 38.5%. The univariate analysis identified a relationship between mortality and the following variables (Table 3): age, length of stay, Ranson score, APACHE II score at admission, APACHE II score at surgery, hematocrit on day 1, Atlanta 2012 classification [4], concurrent multiple organ failure, renal failure, respiratory failure, cardiovascular failure, and antibiotic therapy. The multivariate analysis revealed that mortality was independently correlated with age, length of stay, Ranson score, APACHE II scores at admission and surgery, hematocrit, and renal, respiratory, and cardiovascular failure (Table 4). Multiple organ failure, the Atlanta 2012 classification, and antibiotic therapy were not independently associated with mortality. Thus, the receiver operating characteristic curves were generated to confirm the presence of a significant correlation between mortality and the following factors: age, length of stay, Ranson score, hematocrit and APACHE II score (Figure 1). Patient age showed a clear relationship with mortality; the median age was significantly higher in deceased patients compared with surviving patients (68 years versus 52.5 years, respectively; p = 0.048). However, although the area under the curve (AUC) had an acceptable discriminative power (0.71), it did not allow calculation of a cut-off with acceptable sensitivity and specificity. A similar outcome was observed for the length of stay (AUC = 0.21), APACHE II score at admission (AUC = 0.73), and hematocrit (AUC = 0.683). The shorter length of stay in deceased patients likely reflects their lower survival rate and may not indicate a direct relationship with mortality. The AUC analysis for the Ranson score (AUC = 0.83) and APACHE II at surgery (AUC = 0.92) generated cut-off values with acceptable sensitivity and specificity; therefore, these two parameters served as predictors for mortality in this study. In patients undergoing surgery for severe acute pancreatitis, the Ranson score cut-off was 5 (sensitivity 80% and specificity 61%). The statistical relationship was stronger for the APACHE II score at surgery; a cut-off of 21 allowed for an 86.7% sensitivity and a 91% specificity. The lack of association between multiple organ failure and mortality was unsurprising as multiple organ failure directly depends on individual organ failure [32]. Both individual and multiple organ failure influence mortality. This same phenomenon was observed for multiple organ failure at the Atlanta 2012 consensus. The statistical significance of each individual organ failure (renal, respiratory, and cardiovascular) reflects their high prevalence in deceased patients; respiratory and cardiovascular failure was present in all deceased patients, and renal failure was present in 75% of the deceased. Patients who did not develop these types of organ failure generally survived; only four patients who did not develop renal failure died. A total 53% of patients with respiratory failure died, 64% with renal failure died, and 57% of patients with cardiovascular failure died. The use of prophylactic antibiotics did not influence the incidence of subsequent infection or prognosis. The type of laparotomy was not correlated with the overall rate of complications. However, transverse laparotomy was associated with a higher sequelae rate compared with median laparotomy (54.5% versus 10.7%, respectively). The use of laparostomy did not influence the clinical outcome, the incidence of complications, or the rate of sequelae. The number of procedures was not correlated with the incidence of complications or sequelae. | ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
This study includes several conceptual flaws that should be considered when interpreting the present findings, namely its retrospective nature and small sample size. Another critical limitation in the present study is the absence of cases employing minimally invasive treatment of complicated pancreatitis, which are becoming increasingly commonplace [21] [22] [23]. This case distribution may reflect practices unique to the study locale in which all clinicians within the department treated pancreatitis patients during the study period. This distribution is likely to change as the department has since been segregated into organ-specific subgroups. The descriptive findings of this study are similar to those in previous reports [11] [34]. Notably, the present study is a purely surgical series, which resulted in elevations in several prognostic factors including the Ranson score, APACHE II score, and the CRP. The mean values in the present sample population were well above the cut-offs considered high risk for severe acute pancreatitis [35] [36]. Consequently, the incidence of multiple organ failure is also high. Prophylactic use of antibiotics remains controversial. Although there is no evidence that prophylaxis improves prognosis [6] [12] [16] [37] [38], some reports continue to recommend systemic antibiotic use [6] [12] [38]. This case series occurred during several trends concerning the use and selection of prophylactic antibiotics. Currently, carbapenems and fluoroquinolones are the antibiotics of choice because of their improved ability to penetrate the pancreatic tissue compared with other agents [4] [17]. However, with the high rate of fluoroquinolone resistance that has developed in Escherichia coli in Portugal [39], its use may need to be reconsidered in these cases, as should the use of carbapenems as prophylactic antibiotics. Surgical intervention should be delayed as long as possible [3] [4] [7] [8] [22]. The relationship between early surgery and mortality is evident in several studies [7] [22]. We did not observe this relationship in the current series, but the relationship between the surgical indication and the time of surgery was evident. Patients with abdominal compartment syndrome or multiple organ failure generally underwent surgery sooner. Before the Atlanta 2012 guidelines emerged, severe acute pancreatitis with organ failure at presentation was described as critical acute pancreatitis in several reports [40] [41] because of its high mortality rate. This designation did not persist, but the term remains important regardless. Notably, the rate of patients with infectious pancreatitis who underwent surgery early during the disease course was 41.6%; this trend contradicts earlier studies, which report that infection typically appears late in the disease course, usually after the 3rd week [22] [31] [42]. One of the interesting trends revealed in this study is in the comparison between laparotomy techniques; the present findings could serve as a springboard for a prospective study examining the differing outcomes between median and transverse laparotomy. Based on several studies by Leppäniemi concerning the utility of transverse laparotomy [43] [44] , we began employing the technique and favored some of its advantages, particularly the good access to the pancreatic fossa. However, data in this series showed greater association with sequelae, though the rate of complications did not increase. The rate of complications in this series is similar to those previously described [11] [34], particularly the long hospital and ICU length of stay. The mortality rate is slightly higher than those in recent reports [11] [21]. Recent studies that employ combined surgical approaches [21] (i.e., minimally invasive procedure followed by combined or open technique if indicated - step up approach) report better outcomes. As already discussed, the present series did not include cases employing minimally invasive procedures, which we suspect underlies the comparatively higher mortality in the current population. Most notably, the current findings validate the Ranson score and APACHE II score as prognostic factors, especially the APACHE II score at surgery. There are no known studies describing the prognostic significance of the APACHE II score at surgery, at least none reporting the level of statistical significance observed in this series. The relationship between prognosis, multiple organ failure, and respiratory, circulatory, and renal failure is well documented in surgically and medically treated patients. These factors share an interconnected relationship with systemic inflammatory response syndrome and sepsis that is undoubtedly multifactorial. However, it should be emphasized that the absence of multiple organ failure is, in fact, related with patient's survival. This facet led to the addition of a second classification of severity in acute pancreatitis, known as moderate acute pancreatitis, which is reserved for cases with local complications or temporary organ failure [4] . | ||||||
|
Conclusion
| ||||||
|
Findings are similar to those found in previous reports. The Ranson score and APACHE II (acute physiology and chronic health evaluation II) scores are good prognostic factors for severe acute pancreatitis. Moreover, in this series, APACHE II at surgery appears to be the best predictor of mortality; a cut-off of 21 allowed for an 86.7% sensitivity and a 91% specificity. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Jorge Pereira – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Júlio Constantino – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Liliana Duarte – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Helena Pinho – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Luis Pinheiro – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Jorge Pereira et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|